Hypertrophic cardiomyopathy (HC) is a disease that mainly affects the left ventricle (LV), however recent studies have suggested that it can also be associated with right ventricular (RV) dysfunction. The objective of this study was to determine the prevalence of RV dysfunction in patients with HC and its relation with LV function and outcome. A total of 324 consecutive patients with HC who received care at Stanford Hospital from 1999 to 2012 were included in the study. A group of 99 prospectively recruited age- and gender-matched healthy volunteers were used as controls. RV function was quantified using the RV fractional area change, tricuspid annular plane systolic excursion (TAPSE), and RV myocardial performance index (RVMPI). Compared with the controls, the patients with HC had a higher RVMPI (0.51 ± 0.18 vs 0.25 ± 0.06, p <0.001) and lower TAPSE (20 ± 3 vs 24 ± 4, p <0.001). RV dysfunction based on an RVMPI >0.4 and TAPSE <16 mm was found in 71% and 11% of the HC and control groups, respectively. Worst LV function and greater pulmonary pressures were independent correlates of RV dysfunction. At an average follow-up of 3.7 ± 2.3 years, 17 patients had died and 4 had undergone heart transplantation. LV ejection fraction <50% and TAPSE <16 mm were independent correlates of outcome (hazard ratio 3.98, 95% confidence interval 1.22 to 13.04, p = 0.02; and hazard ratio 3.66, 95% confidence interval 1.38 to 9.69, p = 0.009, respectively). In conclusion, RV dysfunction based on the RVMPI is common in patients with HC and more frequently observed in patients with LV dysfunction and pulmonary hypertension. RV dysfunction based on the TAPSE was independently associated with an increased likelihood of death or transplantation.
Hypertrophic cardiomyopathy (HC) is a genetic disease associated with left ventricular (LV) hypertrophy, which commonly involves the left ventricular septum. Recent studies have shown that cardiac hypertrophy is not limited to the left ventricle. In a magnetic resonance study by Maron et al, the right ventricular (RV) wall thickness was increased in patients with HC compared with controls. To date, however, only limited data are available on the prevalence and clinical correlates of RV dysfunction in patients with HC. In the present study, we sought to determine the prevalence of RV dysfunction in a large series of patients with HC, using 3 echocardiographic indexes: the tricuspid annular plane systolic excursion (TAPSE), RV fractional area change, and RV myocardial performance index (RVMPI). We also sought to determine which factors were associated with a higher incidence of RV dysfunction. Finally, our last objective was to determine in an exploratory analysis whether RV systolic dysfunction was independently associated with an increased likelihood of death or transplantation.
Methods
From January 1999 to January 2012, 434 consecutive patients with HC were enrolled in the Stanford Inherited Cardiomyopathy Registry. In the present study, we retrospectively analyzed 324 cases in which the calculation of the MPI of both ventricles was feasible. The patients were enrolled in the study at their first echocardiogram. We excluded from our analysis technically difficult studies, examinations that did not allow an assessment of the MPI, patients in atrial fibrillation, and those in paced rhythm (because an MPI evaluation in these 2 situations has been considered to be unreliable). The diagnosis of HC was determined by the presence of significant LV hypertrophy (end-diastolic wall thickness ≥15 mm at M-mode or 2-dimensional echocardiography) in the absence of other etiologies, according to international criteria, or a wall thickness of 13 to 15 mm, in presence of abnormal electrocardiographic findings, or a familial history of inherited cardiomyopathy. Patients with LV systolic dysfunction at enrollment were included in the present study if they had a clear documented history of HC and preserved LV ejection fraction on previous echocardiographic examinations performed at other institutions. The patients were medically treated with β blockers, calcium channel blockers, antiarrhythmic agents, and diuretics, as clinically indicated. Implantable cardioverter-defibrillators were implanted for primary or secondary prevention of sudden cardiac death. The investigation conformed with the principles outlined in the Declaration of Helsinki and with the local legal requirements. The institutional review board at Stanford University approved the study.
The control population included 99 prospectively recruited age- and gender-matched subjects. A comprehensive health questionnaire was administered to patients to exclude any symptoms suggestive of cardiovascular disease. We also excluded patients with a family history of dilated cardiomyopathy or coronary artery disease younger than 55 years old. A control group was included for the purposes of comparing the echocardiographic indexes in patients with HC. Echocardiographic images were acquired using a Philips iE33 (iE33 Philips Medical Systems, Andover, Massachusetts). From the echocardiographic findings, ventricular morphology was classified as (1) reverse curve HC, (2) hypertrophied with proximal septal bulge (sigmoid), (3) apical, (4) hypertrophied with a normal shape (symmetric), or (5) neutral, if the morphology did not match the 4 previous subgroups. Using M-mode and 2-dimensional echocardiography, we measured the LV diameter, thickness of the interventricular septum and LV posterior wall, and left atrial end-systolic diameter according to the recommendations of the American Society of Echocardiography ( Figure 1 ). The LV ejection fraction was assessed from the apical 4-chamber view, using the biplane method of discs. The LV ejection fraction was considered reduced if it was <50% (this threshold was defined at the beginning of the study).

At Doppler examination, the systolic intraventricular gradient was quantified using the continuous Doppler technique. A peak gradient >30 mm Hg at rest or after dynamic maneuvers was considered significant. Mitral regurgitation severity was assessed according to the American Society of Echocardiography guidelines. LV filling was assessed by pulsed Doppler interrogation at the level of the mitral opening tips. The pattern of LV filling was classified as a restrictive filling pattern in the presence of an E-deceleration time <120 ms or an E/A wave of ≥2 associated with an E-deceleration time ≤150 ms; abnormal relaxation in the presence of an E/A wave <1 associated with an E-deceleration time >220 ms; or normal (or “pseudo-normal”) in the presence of an intermediate filling pattern.
For tissue Doppler images, we measured the peak myocardial early diastolic velocity at the lateral mitral annulus and transmitral inflow to determine the E/e’. The LVMPI was measured from the mitral inflow and LV outflow Doppler tracings. The RVMPI was calculated using the pulmonary flow velocity and tricuspid inflow velocities ( Figure 2 ). MPI is a Doppler-derived interval index that combines both systolic and diastolic cardiac performance. The MPI is derived using pulsed Doppler echocardiography, as previously described by Tei et al. The RVMPI avoids the geometric assumptions and limitations of complex RV geometry. It is measured as the ratio of the isovolumetric contraction and relaxation time over the ejection time. In previously reported series, the mean normal value of the MPI index was 0.39 ± 0.05 for the left ventricle and 0.28 ± 0.04 for the right ventricle. The upper reference limit for the RVMPI was 0.40 using pulsed Doppler and 0.55 using tissue Doppler in the most recent guidelines of the American Society of Echocardiography. A greater RVMPI implies worse dysfunction. TAPSE was measured from the systolic excursion of the tricuspid lateral annulus using a 2-dimensional method ( Figure 1 ). According to the American Society of Echocardiography guidelines, RV systolic dysfunction should be considered present with a TAPSE <16 mm or fractional area change (apical 4-chamber view) <35%.
Patients were periodically followed up with clinical and/or laboratory assessments. The frequency of evaluations was established according to the clinical needs of the patients. The end point of the study was overall mortality or heart transplantation. The results are presented as the mean ± SD for continuous variables and the number of cases and percentages for categorical variables. A comparison between groups was made using analysis of variance for the continuous variables, using the Brown-Forsythe statistic when the assumption of equal variances did not hold, and the chi-square test for discrete variables, using Yates’ correction when necessary. Multiple regression analysis was used to determine the factors independently associated with RV function. Cumulative event rates were calculated according to the Kaplan-Meier method, with all-event or censoring times measured from the point of enrollment. The significance of differences in mortality between the 2 groups was assessed using the log-rank test. The Cox proportional hazard model was used to determine the factors independently associated with a greater likelihood of death or transplantation. The multivariate model included all the variables that were significant on univariate analysis, as well as age and gender. To determine the intra- and inter-reader variability, the studies were blindly read by the same investigator and by a separate investigator. A random sample of 25 studies was chosen to calculate the intra- and inter-reader variability. The intra- and inter-reader variability were quantified using the mean differences and the intraclass correlation coefficient, and no significant difference (p = 0.86) between the 2 readers was observed. Statistical analysis was performed using PASW software, version 18.0 (PASW, Chicago, Illinois).
Results
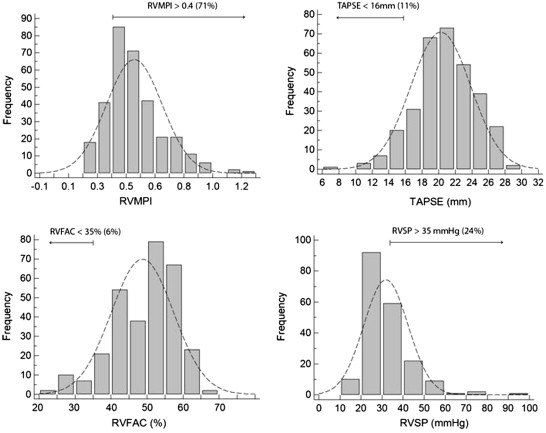
The clinical and basic echocardiographic characteristics of the patients and controls are listed in Tables 1 and 2 . The mean age of the patients with HC was 50 ± 16 years, and 191 (59%) were men. A familial history of sudden death was found in 15%, and LV outflow tract obstruction at rest was present in 33%. The patients were treated mainly with β blockers (56%) and calcium channel blockers (23%). Compared with the controls, the patients with HC had a significantly increased LV wall thickness (interventricular septum 17 ± 5 vs 7 ± 1 mm, p <0.001, posterior wall 12 ± 3 vs 7 ± 1 mm, p <0.001), a smaller LV end-diastolic dimension (42 ± 7 vs 50 ± 5 mm, p <0.001), greater LV ejection fraction (67 ± 10% vs 65 ± 6%, p = 0.0091), and larger left atrium (left atrial volume 80 ± 35 vs 50 ± 20 cm 3 , p <0.001). Considering the parameters of RV function, the TAPSE was significantly lower in the patients with HC (20 ± 3 vs 24 ± 4, p <0.001), and the RV fractional area change was greater (48 ± 9 vs 45 ± 7, p <0.001). The RVMPI was significantly greater in the patients with HC (0.51 ± 0.18 vs 0.25 ± 0.06, p <0.001). The distribution of RV function parameters in the HC population is shown in Figure 2 . The thresholds of abnormal values were determined from the American Society of Echocardiography guidelines of the evaluation of RV function. An abnormal RVMPI (>0.4) was present in 71% of the patients with HC, a TAPSE <16 mm was found in 11%, an RV fractional area change of <35% was present in 6%, and an RV systolic pressure of >35 mmHg was observed in 24% of the patients. A reliable assessment of the RV systolic pressure with a clear modal frequency was possible for 62% of the patient population. The variables that correlated independently with RV dysfunction according to an RVMPI >0.4 were LVMPI (odds ratio [OR] 3.13 per 0.3 u, 95% confidence interval [CI] 1.63 to 6.0, p <0.001) and pulmonary arterial pressure (OR 1.65 per 10 mm Hg, 95% CI 1.08 to 2.53, p = 0.021). Most patients with RV dysfunction according to the RVMPI (82%) had evidence of either LV dysfunction according to the LVMPI or a pulmonary pressure >35 mmHg. The univariate analysis results are listed in Table 3 . Figure 3 shows the association between RVMPI and various LV variables (morphology and LV systolic and diastolic dysfunction) and the correlation between the LVMPI and RVMPI (r = 0.35, p <0.001). The variables independently associated with TAPSE <16 mm were LV ejection fraction <50% (OR 24.9, 95% CI 6.8 to 92, p <0.001) and RV systolic pressure >35 mm Hg (OR 3.3, 95% CI 1.3 to 8.7, p = 0.014). Most patients with RV dysfunction according to the TAPSE (67%) had evidence of either LV dysfunction according to LV ejection fraction or a pulmonary pressure >35 mm Hg. The variables considered in the model were age, gender, LV ejection fraction <50%, restrictive filling pattern, LVMPI, RV systolic pressure >35 mm Hg, and left atrial volume.
| Variable | Patients With HC (n = 324) |
|---|---|
| Age (yrs) | 50 ± 16 |
| Male gender | 191 (59) |
| Septal reverse morphology | 170 (52) |
| Septal sigmoid morphology | 36 (11) |
| Symmetric morphology | 55 (17) |
| Pure apical morphology | 12 (4) |
| Neutral morphology | 51 (16) |
| Family history of HC | 111 (34) |
| Family history of sudden death | 50 (15) |
| History of syncope | 49 (15) |
| CAD | 10 (3) |
| Mild hypertension | 31 (10) |
| NYHA class III-IV | 37 (11) |
| LVEF (%) | 67 ± 10 |
| LV restrictive filling pattern | 18 (6) |
| LV gradient >30 mm Hg at rest | 108 (33) |
| Moderate to severe MR | 17 (5) |
| LVEF <50% | 17 (5) |
| β Blockers | 182 (56) |
| Calcium channel blocker | 73 (23) |
| ACE inhibitors | 57 (18) |
| Diuretics | 43 (13) |
| Antiarrhythmic therapy | 14 (4) |
| ICD | 99 (31) |
| Appropriate ICD discharge during follow-up | 23 (7) |
| Variable | HC Group (n = 324) | Control Group (n = 99) | p Value |
|---|---|---|---|
| Age (yrs) | 50 ± 16 | 51 ± 16 | 0.59 |
| Male gender | 191 (59%) | 59 (59%) | 1 |
| TAPSE (mm) | 20 ± 3 | 24 ± 4 | <0.001 |
| RVFAC (%) | 48 ± 9 | 45 ± 7 | <0.001 |
| RVFAC <35% | 16 (5%) | 0 | 0.02 |
| RA volume (cm 3 ) | 45 ± 20 | 41 ± 19 | 0.12 |
| RVMPI | 0.51 ± 0.18 | 0.25 ± 0.06 | <0.001 |
| RSVP (mm Hg, n = 201) | 32 ± 10 | 14 ± 9 | <0.001 |
| OR (95% CI) | p Value | |
|---|---|---|
| Age (per 10 yrs) | 1.18 (1.01–1.37) | 0.036 |
| Male gender | 1.20 (0.73–1.98) | 0.46 |
| LVEF (per decrease 10%) | 1.35 (1.04–1.71) | 0.022 |
| LVMPI (per 0.3) | 2.53 (1.65–3.89) | <0.001 |
| E/e′ (per 5 units) | 1.19 (0.97–1.48) | 0.09 |
| IVS thickness (per 10 mm) | 1.04 (0.67–1.62) | 0.86 |
| IVS >30 mm | 1.26 (0.34–4.71) | 0.73 |
| PW thickness (per 5 mm) | 1.71 (1.16–2.52) | 0.007 |
| LV gradient >30 mm Hg at rest | 0.68 (0.41–1.16) | 0.16 |
| Moderate to severe MR | 3.06 (0.69–13.67) | 0.14 |
| LA volume (per 10 cm 3 ) | 1.05 (0.98–1.13) | 0.15 |
| RVSP (per 10 mm Hg) | 1.64 (1.16–2.32) | 0.005 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


