Predisposing risk factors, clinical course, and prognosis of spontaneous coronary artery dissection (SCAD) remain poorly understood. We reviewed medical records and coronary angiograms of patients admitted to our institution with the diagnosis of SCAD from 1999 through 2010. A definite diagnosis of SCAD required the agreement of 2 blinded board-certified interventional cardiologists who reviewed all images separately. Baseline characteristics of patients (n = 23) included mean age 45 ± 11 years, female gender in all (100%), history of hypertension in 13 (57%), and postpartum in 7 (30%). Eleven (48%) had ST-segment elevation on initial electrocardiogram. SCAD involved the left main in 5 patients (21.7%), left anterior descending coronary artery in 16 (70%), left circumflex coronary artery in 8 (35%), and right coronary artery in 6 (26%). Four patients (17%) underwent coronary stenting and 6 (26%) required urgent bypass surgery. Comparison between postpartum and nonpostpartum patients revealed significant differences in mean peak troponin levels: 50 ± 34 ng/ml vs 21 ± 23, p = 0.04, mean left ventricular ejection fraction: 34 ± 6% vs 49 ± 9, p <0.01, proximal coronary segment distribution: 6 (86%) vs 3 (19%), p = 0.004, and left anterior descending coronary artery distribution: 7 (100%) vs 9 (56%), p = 0.04, respectively. Repeat coronary angiographies were performed in 11 patients (46%) during a mean follow-up of 39 ± 38 months and 10 (91%) were found to have healed SCAD, including those who had undergone bypass surgery. In conclusion, our patients with SCAD were characterized by female gender, absence of coronary risk factors, and a high rate of vascular healing without residual stenosis. Larger infarct was found in postpartum patients.
We investigated clinical characteristics of patients admitted to our institution with a diagnosis of spontaneous coronary artery dissection (SCAD). Individual cases are briefly described, and coronary angiograms and intravascular ultrasound (IVUS) images are shown. We highlight the diagnosis and management challenges that these patients represent in contemporary practice.
Methods
We retrospectively reviewed medical records and coronary angiograms of patients admitted to our institution with a diagnosis of SCAD from July 1999 through March 2010.
A diagnosis of SCAD is often challenging because angiographic features seen in SCAD may be extremely heterogenous. In this study SCAD was suspected on coronary angiogram if there was a characteristic finding suggesting the presence of coronary artery dissection such as a visible intraluminal filling defect, extraluminal opacity or flap on coronary angiograms, or a visible intramural hematoma or flap on IVUS images. A definite diagnosis of SCAD was given if there was unanimous agreement of 2 blinded board-certified interventional cardiologists who reviewed all images separately.
Postpartum SCAD was defined if SCAD was diagnosed in a patient with a documented pregnancy within the previous 5 months. Myocardial infarction was defined according to universal definition published elsewhere.
Baseline characteristics and clinical outcomes are presented and followed by a comparison of postpartum versus nonpostpartum cases. Continuous variables are expressed as mean ± standard deviation (SD) and compared using t test. Categorical variables are expressed as percentage and compared using chi-square test. In all tests a 2-sided p value <0.05 was accepted as a statistically significant difference. All statistics analyses were performed using STATA 10 (STATA Corporation LP, College Station, Texas).
Results
We found 31 patients who were admitted to our institution with a diagnosis of SCAD from July 1999 through March 2010. After reviews of angiograms by 2 blinded interventional cardiologists and their agreements, 23 patients with definite SCAD were identified. Baseline characteristics and clinical courses of individual cases are briefly listed in Table 1 .
| Case | Age (years) | Race | Postpartum | Presentation | Coronary Arteries Involved on First Angiogram ⁎ | Coronary Arteries Involved on Second Angiogram † | Nonmedical Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 30 | White | Yes (4 weeks) | STEMI | LAD | LAD | |
| 2 | 30 | Black | Yes (14 days) | NSTEMI | LAD, LCx | LAD, LCx | |
| 3 | 35 | Black | Yes (19 days) | UAP | LAD, LCx | LAD, LC, RCA | CABG |
| 4 | 36 | Hispanic | Yes (7 days) | STEMI | LAD | Failed rheolytic thrombectomy | |
| 5 | 37 | White | Yes (5 months) | NSTEMI | LAD | CABG | |
| 6 | 39 | White | Yes (12 days) | STEMI | LAD | LM, LAD, LCx | CABG |
| 7 | 39 | White | Yes (10 days) | NSTEMI | LAD | LAD | Stent |
| 8 | 36 | White | No | NSTEMI | LAD | CABG | |
| 9 | 38 | White | No | NSTEMI | LCx | ||
| 10 | 38 | White | No | STEMI | LM, LAD, LCx | CABG | |
| 11 | 40 | White | No | STEMI | LAD, LCx | Thrombolysis | |
| 12 | 40 | White | No | STEMI | LAD | LAD | Stent |
| 13 | 41 | White | No | NSTEMI | LAD | LAD | |
| 14 | 45 | White | No | STEMI | RCA | ||
| 15 | 45 | White | No | STEMI | RCA | Thrombolysis | |
| 16 | 51 | White | No | NSTEMI | LAD, RCA | LM, LAD, RCA | Stent, CABG |
| 17 | 52 | C | No | STEMI | LAD | ||
| 18 | 54 | White | No | STEMI | LAD | ||
| 19 | 56 | White | No | NSTEMI | RCA | ||
| 20 | 57 | White | No | NSTEMI | LC | ||
| 21 | 62 | C | No | NSTEMI | RCA | ||
| 22 | 64 | White | No | STEMI | LAD | ||
| 23 | 66 | AA | No | NSTEMI | LCx | LCx (extension) | Stent |
† Second-look angiogram or angiogram after percutaneous coronary intervention.
Baseline characteristics of patients are listed in Table 2 . Notably all patients (n = 23, 100%) identified with definite SCAD were women. Aside from hypertension, prevalence of traditional coronary risk factors was low. Seven patients (30.4%) in our series developed SCAD postpartum (vaginal delivery in 5 patients, cesarean section in 2) and none (0%) was pregnant. Compared to other patients, postpartum patients were younger and more likely non-Caucasian, although mean age (35 ± 4 years) was relatively advanced for pregnancy.
| Variables | Postpartum (n = 7) | Nonpostpartum (n = 16) | p Value |
|---|---|---|---|
| Age (years), mean ± SD | 36 ± 4 | 49 ± 10 | <0.01 |
| Race | 0.09 | ||
| Caucasian | 4 (57%) | 15 (94%) | |
| African-American | 2 (29%) | 1 (6%) | |
| Hispanic | 1 (14%) | 0 | |
| History of smoking | 2 (29%) | 5 (31%) | 0.90 |
| Hypertension | 1 (14%) | 12 (75%) | 0.07 |
| Diabetes mellitus | 1 (14%) | 0 | 0.12 |
| Hyperlipidemia | 0 | 5 (31%) | 0.10 |
| Previous cardiac disease | 0 | 0 | N/A |
| Connective tissue disorders | 0 | 0 | N/A |
| Use of oral contraceptives | 0 | 2 (13%) | 0.33 |
Clinical presentations are presented in Table 3 . All patients (n = 23, 100%) presented with an acute coronary syndrome. Of these there were increases of troponin I levels (myocardial infarction) in 22 patients excluding a patient whose cardiac enzyme level was not found in medical records. Emotional stress before the symptom was identified in 4 patients (17%). The most striking case was a patient (case 10) who developed chest pain immediately after learning that her husband wanted a divorce. This particular coronary angiogram ( Figure 1 ) revealed extensive coronary dissections involving the left main coronary artery, left anterior descending coronary artery (LAD), and left circumflex coronary artery, which led to emergency coronary artery bypass grafting (CABG). The patient in case 9 presented with SCAD after sustaining left-sided chest trauma from a water-skiing accident. Table 3 presents more prevalent congestive heart failure and higher mean value of peak troponin I levels in postpartum patients.
| Study Variables | Postpartum (n = 7) | Nonpostpartum (n = 16) | p Value |
|---|---|---|---|
| Chest pain | 7 (100%) | 16 (94%) | N/A |
| Dyspnea | 4 (57%) | 4 (25%) | 0.14 |
| Cardiogenic shock | 1 (14%) | 0 | 0.12 |
| Congestive heart failure on presentation | 2 (29%) | 0 | 0.03 |
| Coincided trauma | 0 | 1 (6%) | 0.50 |
| Emotional stress identified | 0 | 4 (25%) | 0.13 |
| Median postpartum days | 19 | N/A | N/A |
| ST-segment elevation on electrocardiogram | 4 (57%) | 7 (44%) | 0.55 |
| Increased troponin I levels | 6 (86%) | 16 (100%) | 0.12 |
| Peak troponin I levels (ng/ml), mean ± SD | 50 ± 34 | 21 ± 23 | 0.04 |
| Location of spontaneous coronary artery dissection | |||
| Left main coronary artery | 3 (43%) | 2 (13%) | 0.10 |
| Left anterior descending coronary artery | 7 (100%) | 9 (56%) | 0.04 |
| Left circumflex coronary artery | 3 (43%) | 5 (31%) | 0.59 |
| Right coronary artery | 1 (14%) | 5 (31%) | 0.35 |
| Proximal segments in any vessel | 6 (86%) | 3 (19%) | <0.01 |
| ≥2 coronary trees involved (%) | 3 (43%) | 3 (19%) | 0.23 |
| Left ventricular ejection fraction (%), mean ± SD | 34 ± 6 | 49 ± 9 | <0.01 |
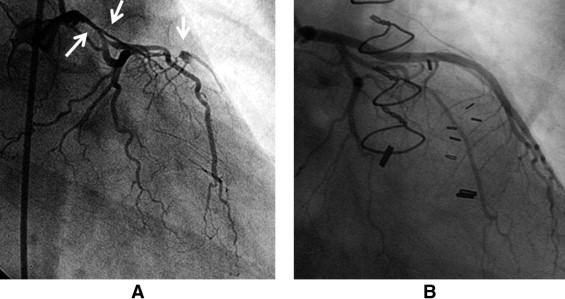
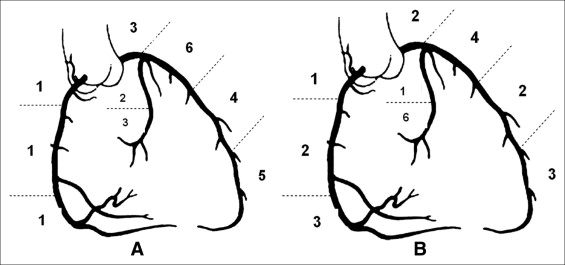
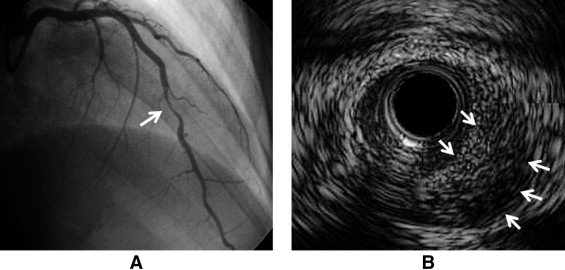
Angiographic findings are presented in Figure 2 and Table 3 . SCAD was seen most in the LAD. Two patients (9%) with ambiguous angiographic findings were further evaluated by IVUS ( Figure 3 ), which confirmed the diagnosis of SCAD. Specific comparisons between postpartum and nonpostpartum cases revealed statistically significantly more involvement of the LAD and proximal segments on angiograms and lower left ventricular ejection fraction on echocardiograms in postpartum patients.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


