6 Preoperative Coronary Intervention
 Overview of Perioperative MI
Overview of Perioperative MI
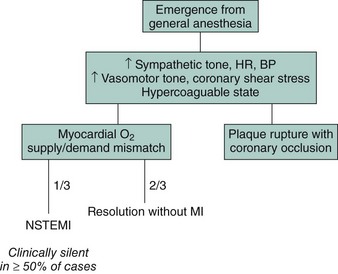
As with MI outside the context of surgery, perioperative MI can result either from the rupture of an atherosclerotic plaque with thrombotic occlusion of the involved coronary artery or from a transient stress-induced mismatch of myocardial oxygen supply and demand, typically in the setting of a fixed coronary artery stenosis or occlusion.1 Thrombosis of a previously implanted coronary stent represents another potential mechanism of perioperative MI and has been described among patients undergoing noncardiac surgery early following BMS implantation and up to several years after DES placement. In the vast majority of instances, perioperative MI does not occur during the surgical procedure itself but rather within the first 48 hours following surgery. This period is associated with multiple hemodynamic stresses and hematological alterations that can predispose to plaque rupture, myocardial oxygen supply-demand mismatch, and a hypercoagulable state (Fig. 6-1). The incidence of myocardial ischemia and infarction in the period surrounding noncardiac surgery is a function of both the type of surgery and the risk profile of the population being studied. The ACC/AHA guidelines for perioperative assessment classify specific operations as high risk (>5% reported rate of cardiac death and nonfatal MI), intermediate risk (1%–5%), or low risk (<1%) (Table 6-1).2 Perioperative MI, whether clinically apparent or silent, is associated with increased mortality in both the short and long term. In-hospital survival rates for perioperative MI typically exceed 90%, which is similar to that of MI in the nonoperative setting. As with clinically silent MI detected following PCI, even smaller infarcts detected following noncardiac surgery are associated with up to a two- to fourfold increase in medium- to late-term mortality independent of other clinical factors.3,4
TABLE 6-1 Cardiac Risk* for Noncardiac Surgical Procedures
| Vascular (reported cardiac risk often greater than 5%) |
| Intermediate (reported cardiac risk generally less than 5%) |
| Low (reported cardiac risk generally less than 1%) |
From The ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery.2
Determining Operative Risk
Concepts
The guidelines recommend a stepwise approach in determining the need for invasive testing prior to noncardiac surgery. First, patient-specific risk is determined through the assessment of clinical risk factors and symptoms, overall functional capacity, and the timing and results of prior coronary evaluation and treatment if applicable. Second, surgery-specific cardiac risk is determined based on the expected incidence of cardiac events associated with the particular surgery the patient is scheduled to undergo, as previously discussed. The decision of whether to perform noninvasive stress testing is then based on assessment of the patient and surgery-specific risks. Despite the comprehensive nature of the ACC/AHA preoperative guidelines, adherence to the guidelines in “real-world” clinical practice is highly variable.5
Indications for Coronary Angiography
The indications for performing coronary angiography as part of a preoperative assessment are identical to those in the nonoperative setting, and include the following2,6:
 Pharmacological Therapy
Pharmacological Therapy
Beta Blockers
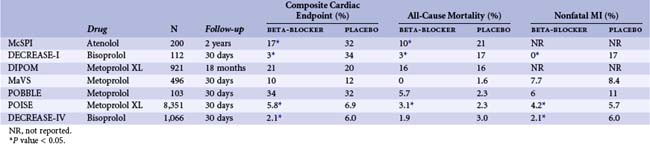
Beta blockers are thought to ameliorate cardiac stress in the perioperative setting by a number of mechanisms, including reduction of adrenergic stimulation, improvements in the balance of myocardial oxygen demand and supply, diminution of arrhythmic potential, and potential anti-imflammatory and plaque-stabilizing effects. Based on the results of two relatively small trials published in the 1990s demonstrating significant reductions in mortality associated with perioperative beta-blocker therapy among patients at risk for coronary events, guidelines advocating routine beta-blocker administration for patients at risk for cardiac complications or undergoing higher-risk surgical procedures became widely adopted. Several ensuing trials failed to demonstrate benefit from routine perioperative beta-blocker therapy among higher-risk patients, raising questions regarding therapeutic efficacy (Table 6-2). Publication of the more recent DECREASE-IV (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo) and POISE (Patients Undergoing Non-Cardiac Surgery) trials has provided new insights while simultaneously raising new questions regarding the role of perioperative beta-blocker therapy.7,8 The DECREASE-IV trial, which demonstrated a significant reduction in the incidence of cardiac death or nonfatal MI associated with perioperative bisoprolol therapy among patients at intermediate cardiac risk undergoing noncardiac surgery, appeared to settle the controversy in favor of beta-blocker therapy. In DECREASE-IV, bisoprolol was initiated at a low dose 30 days prior to surgery and carefully titrated to a goal heart rate of 50 to 70 beats per minute in 1,066 patients. Conflicting findings from the much larger POISE trial, however, again raised doubts regarding the safety and effectiveness of routine perioperative beta-blocker use. In POISE, 8,351 patients at risk for or with known coronary artery disease were assigned to receive either extended-release metoprolol or placebo beginning 2 to 4 hours before noncardiac surgery and continued for 30 days. At 30-day follow-up, the incidence of the composite primary endpoint of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest was significantly lower among those assigned to metoprolol (5.8% vs. 6.9%), yet this advantage was offset by significantly increased frequencies of both all-cause mortality and stroke among individuals treated with beta blockers rather than placebo.
Many observers believe that the discordant results of trials examining perioperative beta blockade are multifactorial and likely attributable to differences with respect to the baseline cardiac risk of the populations under study, the particular beta blocker used, and whether therapy was begun well in advance of surgery and titrated to effect or simply started at a fixed uniform dose a few hours before the operation.9,10 Based on the latest study data, the ACC/AHA established updated guidelines in 2009 for the use of beta blockers for noncardiac surgery. The guideline statement concludes that while the most recent studies suggest that “beta blockers reduce perioperative ischemia and may reduce the risk of MI and cardiovascular death in high-risk patients, routine administration of higher-dose long-acting metoprolol in beta-blocker naive patients on the day of surgery and in the absence of dose titration is associated with an overall increase in mortality.” The lone class-I recommendation for beta blockade in the current guidelines states that “beta blockers should be continued in patients undergoing surgery who are [already] receiving beta blockers” for appropriate indications. Class IIa recommendations support the use of beta blockers titrated to heart rate and blood pressure for patients undergoing vascular or intermediate-risk surgery who are at high cardiac risk as a result of either (1) known coronary artery disease, (2) the finding of cardiac ischemia on preoperative testing, or (3) the presence of more than one clinical risk factor for coronary disease. The routine administration of high-dose beta blockers in the absence of dose titration is now contraindicated (class III) for patients undergoing noncardiac surgery who are not currently taking beta blockers.
Statin Therapy
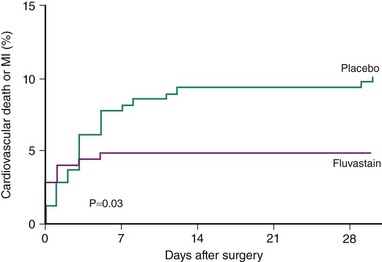
Proposed mechanisms by which statins may reduce perioperative cardiac complications relate to the plaque-stabilizing effects of these agents, including their anti-inflammatory and anti-thrombotic properties, and the favorable influences these drugs exert on plaque-related endothelial dysfunction.11 The withdrawal of statin therapy in the perioperative period has been associated with increased cardiac events.12 Recent randomized trials have supported earlier observational studies suggesting that statin therapy administered prior to noncardiac surgery may be associated with a reduction in major adverse events. The DECREASE-III trial randomized 497 statin-naive patients scheduled to undergo vascular surgery to receive fluvastatin 80 mg daily or placebo started a median of 37 days preoperatively.13 All patients also received titrated beta-blocker therapy. Fluvastatin therapy was associated with significant reductions in the incidence of perioperative myocardial ischemia on continuous ECG monitoring (10.8% vs. 19%, hazard ratio [HR] 0.55; 95% confidence interval [CI], 0.34–0.88) and in 30-day cardiovascular death or MI (HR, 0.47; 95% CI, 0.24–0.94) (Fig. 6-2). In the DECREASE-IV trial, which utilized a 2 × 2 factorial design to study the effects of perioperative fluvastatin and bisoprolol therapy, randomization to fluvastatin 80 mg daily was associated with a nonsignificant trend toward reduced 30-day cardiac events (HR, 0.65; 95% CI, 0.35–1.10).8
 Preoperative Coronary Revascularization
Preoperative Coronary Revascularization
Coronary Artery Bypass Grafting
Noncardiac surgery appears safe for individuals with remote CABG who subsequently require a major noncardiac surgical procedure. Among patients followed in the CASS (Coronary Artery Surgery Study) registry who required high-risk noncardiac surgery during follow-up, prior CABG was associated with reduced postoperative MI and mortality compared with medically managed coronary disease. Other observational reports have indicated that for patients with prior CABG, mortality rates associated with noncardiac surgery are comparable with those observed among patients without evidence of coronary disease. While a history of remote CABG may be protective during future surgical procedures, the role of CABG undertaken as a preemptive measure among patients discovered to have severe coronary artery disease during a preoperative risk assessment remains unproven. Performance of CABG can substantially delay subsequent noncardiac surgery, and prophylactic CABG may not be feasible if the required noncardiac surgical procedure is urgent or semiurgent. Attempts to perform noncardiac surgery very shortly after CABG may be associated with further increased operative risks. For example, in one observational study, patients undergoing vascular surgery within 1 month of CABG demonstrated a fivefold increase in operative mortality compared with matched controls who underwent vascular surgery without preceding CABG (20.6 vs. 3.9%, P < 0.005).14
Percutaneous Coronary Intervention
Observational Studies of Preoperative Percutaneous Coronary Intervention
A variety of retrospective analyses extending from the pre-stent era to current practice have addressed the relationship between preoperative PCI and the likelihood of adverse cardiac events during subsequent noncardiac surgery (Tables 6-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree