Pregnancy in Young Women with Congenital Heart Disease
Candice K. Silversides
Jack M. Colman
Samuel C. Siu
In general, pregnancy is well tolerated in women with congenital heart disease. However, misconceptions are common. Discussions regarding contraception and pregnancy should begin once adolescent women reach an age when they may become sexually active. Optimal management includes a complete cardiac assessment prior to conception. This assessment should include a full review of the underlying cardiac lesions and prior surgical procedures, determination of the risk of pregnancy, and development of plans for cardiac interventions prior to pregnancy when indicated. Because the severity of a low-risk condition may be misinterpreted or given undue importance, even women with low-risk cardiac lesions often benefit from preconception counseling. All women need to understand which types of contraception are appropriate and safe. Unfortunately, among women with congenital heart disease preconception counseling is often not provided and knowledge of risks of contraception and pregnancy is often suboptimal (1,2,3).
Many issues need to be addressed in women with heart disease contemplating or undergoing pregnancy, including the risks for the mother and the baby, possible adverse effects of medication used during pregnancy, maternal long-term prognosis, and the risk of recurrence of cardiac disease in offspring (Table 69.1). The cardiologist must play the critical role of providing and/or ensuring informed education of the patient, her partner, and her caregivers, as other caregivers are less likely to do so.
Physiologic Changes during Pregnancy
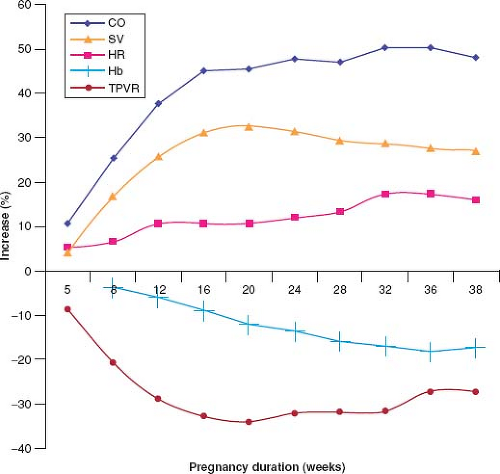
Maintenance of adequate oxygen delivery to maternal peripheral tissues as well as to the fetus is achieved through changes in maternal circulating blood volume, red cell mass, peripheral vascular compliance and resistance, heart rate, and cardiac output (Fig. 69.1) (4). These adaptive changes are usually well tolerated by women without heart disease; however, in some women with heart disease such changes result in cardiac decompensation. As well, pre-existing heart disease may first be revealed during pregnancy when the heart is challenged by an increased hemodynamic burden.
TABLE 69.1 Women of Childbearing Age with Cardiac Disease: Approach to Contraception and Pregnancy | |||
|---|---|---|---|
|
Blood volume increases during pregnancy; the increase begins as early as the sixth week of gestation and peaks at an average of 50% more than the prepregnant state by the end of the second
trimester (5,6), though individual increases range between 20% and 100% above prepregnant blood volume (7). Blood volume plateaus in the third trimester (8). Red cell mass increases during pregnancy to as much as 40% above prepregnancy levels (6,9). A “physiologic anemia of pregnancy” is seen because the increase in plasma volume is proportionately greater than the increase in red blood cell mass. In addition, there are increased levels of clotting factors and decreased fibrinolytic activity (10), both acting to promote the hypercoagulability that underlies the increased risk for thromboembolism during pregnancy.
trimester (5,6), though individual increases range between 20% and 100% above prepregnant blood volume (7). Blood volume plateaus in the third trimester (8). Red cell mass increases during pregnancy to as much as 40% above prepregnancy levels (6,9). A “physiologic anemia of pregnancy” is seen because the increase in plasma volume is proportionately greater than the increase in red blood cell mass. In addition, there are increased levels of clotting factors and decreased fibrinolytic activity (10), both acting to promote the hypercoagulability that underlies the increased risk for thromboembolism during pregnancy.
Systemic (peripheral) vascular resistance decreases beginning in the fifth week of gestation. This mediates a decrease in systemic arterial pressure that begins in the first trimester and reaches its nadir in mid pregnancy, after which blood pressure stabilizes (11,12). After the 32nd week of gestation, the systemic vascular resistance slowly increases until term, accompanied by recovery of systemic arterial pressure, which ultimately reaches or exceeds prepregnancy levels. Renal blood flow also increases, accompanied by a 50% increase in glomerular filtration rate (13). Increased blood flow to the hands and feet, nasal passages, and breasts results in warm erythematous extremities, nasal congestion, and breast engorgement, respectively. The impact of pregnancy on coronary blood flow has not been studied.
Cardiac output increases during pregnancy as a result of increases in both heart rate and stroke volume. Most of the early increase in cardiac output is a result of progressive increase in stroke volume, whereas later in pregnancy the heart rate effect becomes more important because the stroke volume stabilizes while the heart rate continues to rise (11,14). The mean heart rate increases to approximately 10 to 20 beats above prepregnancy levels by term. Increase in cardiac output begins as early as the 5th week of gestation, reaches its zenith near the end of the second trimester, typically after the 24th week of gestation and then plateaus until term at 30% to 50% above prepregnancy levels (11,15,16,17). Pregnant women with underlying cardiac disease have been shown to have lower cardiac output than pregnant women with normal cardiac function (18). Cardiac output can fall acutely if the inferior vena cava is compressed by the gravid uterus in the supine position, a phenomenon that can be reversed by assuming the left lateral decubitus position. Although increases in left ventricular ejection fraction during pregnancy have been reported by some (11,16), other studies have not demonstrated this finding (17,19,20).
During labor and delivery pain, anxiety and uterine contractions result in tachycardia, hypertension, and further increases in cardiac output, sometimes provoking cardiac decompensation in women with heart disease. During labor, there is a 10% increase in cardiac output beyond the pre-labor level, mediated by increases in the heart rate and stroke volume, augmented by yet a further increase of 7% to 15% in response to each uterine contraction, with maximal augmentation noted during the second stage of labor (21). Immediately following delivery, cardiac output may transiently increase to as much as 80% above pre-labor values due to relief of inferior vena cava compression and autotransfusion from the placenta, but output returns to pre-labor levels by approximately 1 hour postpartum. Thereafter, the hemodynamic changes that developed during pregnancy return toward baseline values; most of the changes resolve early after delivery, although complete resolution of all measureable pregnancy-associated effects may take as long as 6 months (22).
Cardiac Findings in Normal Pregnancy
Fatigue, dyspnea, light-headedness, and palpitations are symptoms associated with normal pregnancy but overlap with symptoms of cardiac decompensation. The hemodynamic changes of pregnancy are responsible for corresponding changes in the physical examination
that can mimic cardiac disease. They include displacement of the apical impulse, prominence of the jugular venous pulsation, wide splitting of the first and second heart sounds, soft systolic flow murmurs and continuous murmurs. Sinus tachycardia and premature atrial or ventricular ectopic beats may also increase in frequency during normal pregnancy and do not necessarily reflect cardiac decompensation or any cardiac disease. This overlap of signs and symptoms may make diagnosis of cardiac decompensation during pregnancy challenging; brain natriuretic peptide can be a useful test to adjudicate the basis for symptoms and signs when a benign basis is not certain (23).
that can mimic cardiac disease. They include displacement of the apical impulse, prominence of the jugular venous pulsation, wide splitting of the first and second heart sounds, soft systolic flow murmurs and continuous murmurs. Sinus tachycardia and premature atrial or ventricular ectopic beats may also increase in frequency during normal pregnancy and do not necessarily reflect cardiac decompensation or any cardiac disease. This overlap of signs and symptoms may make diagnosis of cardiac decompensation during pregnancy challenging; brain natriuretic peptide can be a useful test to adjudicate the basis for symptoms and signs when a benign basis is not certain (23).
On the 12-lead electrocardiogram during normal pregnancy, there may be a leftward shift in the frontal axis, T-wave inversion in lead II and ST-segment depression. Echocardiographic studies during normal pregnancy reveal that dimensions of all four cardiac chambers increase and there is an increase in left ventricular wall thickness and mass (16,22,24,25). Increases in transvalvular flow velocities are seen. Mitral, tricuspid, and pulmonic annular diameters increase and may result in increasing degrees of mitral, tricuspid, and pulmonic regurgitation, respectively (26).
Assessment of Pregnancy Risk in Women with Congenital Heart Disease: General Concepts and Global Evaluation
Women with cardiac disease are at increased risk of developing adverse maternal cardiac events during pregnancy (27). Maternal cardiac risk can usually be estimated after a complete cardiovascular history and physical examination, a 12-lead electrocardiogram, a transthoracic echocardiogram, and arterial oxygen saturation when indicated. Echocardiography is safe during pregnancy. Prepregnancy exercise testing, specifically focusing on measures of heart rate responsiveness to exercise, may aid risk stratification (28). Prepregnancy stress testing to assess functional capacity and blood pressure response to exercise can help with risk stratification in women with severe aortic stenosis. Stress echocardiography can be used to assess ischemia in women with coronary anomalies. Women with Marfan syndrome and other hereditable aortopathies should have complete imaging of the aorta prior to pregnancy using magnetic resonance imaging (MRI) or computed tomography (29).
TABLE 69.2 Risk Factors for Adverse Maternal Cardiac and Fetal/Neonatal Adverse Events During Pregnancy in Women with Heart Disease | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
Early studies showed that poor maternal functional class and cyanosis are associated with adverse maternal cardiac events (30,31). Subsequently, a prospective multicenter study addressed global risk assessment of pregnant women with congenital and acquired heart disease and identified poor functional status (NYHA >II) or cyanosis, systemic (subaortic, “left”) ventricular systolic dysfunction, left heart obstruction, and history of cardiac events prior to pregnancy (arrhythmia, stroke, or pulmonary edema) as independent predictors of maternal cardiac events during pregnancy. Based on these predictors, women can be classified into low- (0 predictor), intermediate- (1 predictor), or high- (>1 predictor) risk categories. The study showed that women in low-, intermediate-, and high-risk categories have, respectively, a 5%, 25%, or >75% chances of developing an adverse cardiac event during pregnancy (Table 69.2)
(Fig. 69.2) (32). This risk score, sometimes referred to as the CARPREG score, has been validated by other groups (33). Additional risk predictors have also been identified. A single-center retrospective study that validated the CARPREG index in a population limited to congenital heart disease reported also that subpulmonary ventricular dysfunction and/or severe pulmonic regurgitation were additional independent risk factors for adverse maternal outcomes (33). The ZAHARA investigators carried out a large multicenter retrospective review of outcomes in pregnant women with congenital heart disease (n = 1,302 pregnancies in 714 women) and derived a weighted risk score that included additional factors such as mechanical valve replacement and moderate or severe subaortic (systemic) or subpulmonary atrioventricular valve regurgitation (34) (see Table 69.2). In a single-center retrospective study examining outcomes in 1,741 women, the largest to date, the maternal cardiac event rate was 9.5%. In addition to previously described risk predictors (cardiac events before pregnancy, NYHA class >II, oxygen saturation <90%, cyanotic heart disease without surgical intervention, and reduced left ventricular systolic function), pulmonary artery hypertension was an identified predictor (35). In 2011, ESC guidelines proposed the use of a modified WHO classification that incorporates the published risk factors and expert opinion into four risk levels corresponding to a negligible risk of cardiovascular complications (WHO I) to prohibitively high risk of maternal mortality or severe morbidity (WHO IV) (Table 69.3) (29).
(Fig. 69.2) (32). This risk score, sometimes referred to as the CARPREG score, has been validated by other groups (33). Additional risk predictors have also been identified. A single-center retrospective study that validated the CARPREG index in a population limited to congenital heart disease reported also that subpulmonary ventricular dysfunction and/or severe pulmonic regurgitation were additional independent risk factors for adverse maternal outcomes (33). The ZAHARA investigators carried out a large multicenter retrospective review of outcomes in pregnant women with congenital heart disease (n = 1,302 pregnancies in 714 women) and derived a weighted risk score that included additional factors such as mechanical valve replacement and moderate or severe subaortic (systemic) or subpulmonary atrioventricular valve regurgitation (34) (see Table 69.2). In a single-center retrospective study examining outcomes in 1,741 women, the largest to date, the maternal cardiac event rate was 9.5%. In addition to previously described risk predictors (cardiac events before pregnancy, NYHA class >II, oxygen saturation <90%, cyanotic heart disease without surgical intervention, and reduced left ventricular systolic function), pulmonary artery hypertension was an identified predictor (35). In 2011, ESC guidelines proposed the use of a modified WHO classification that incorporates the published risk factors and expert opinion into four risk levels corresponding to a negligible risk of cardiovascular complications (WHO I) to prohibitively high risk of maternal mortality or severe morbidity (WHO IV) (Table 69.3) (29).
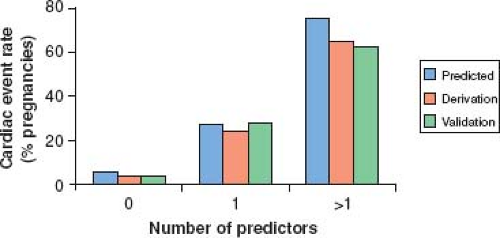 Figure 69.2 Frequency of adverse maternal cardiac events in pregnancy as predicted by the CARPREG risk index. Frequency of primary maternal cardiac events, as predicted by the CARPREG risk index (32), and observed in derivation and validation groups, expressed as a function of the number of predictors (x-axis). (From Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–521.) |
Any global risk index should be used in conjunction with lesion-specific risk estimates, since certain intermediate- or high-risk lesions may not have been represented in the population from which the global risk index was derived. For example, women with Marfan syndrome and dilated aortic root, Eisenmenger syndrome, or those with a Fontan circulation were underrepresented in the derivation sets and therefore their known pregnancy-associated risks will not be reliably predicted by the global risk scores. The various risk indices are helpful in placing patients into risk groups, but clinical judgment and expertise are required to fine tune risk stratification. Furthermore, the prediction rules reported discriminative (differentiate between those that will vs. will not develop complications) and calibrative (agreement between expected vs. observed rates of complications within each risk level) accuracies may be different when applied to populations with different lesion mix, healthcare environment, or earlier era (36,37,38). At our center, we use global risk scores to place patients into low-, intermediate-, and high-risk groups, avoiding quantifying risk numerically in view of the previous-mentioned limitations.
TABLE 69.3 Modified WHO Classification of Maternal Cardiovascular Risk in Women with Congenital Heart Disease | ||||||
|---|---|---|---|---|---|---|
|
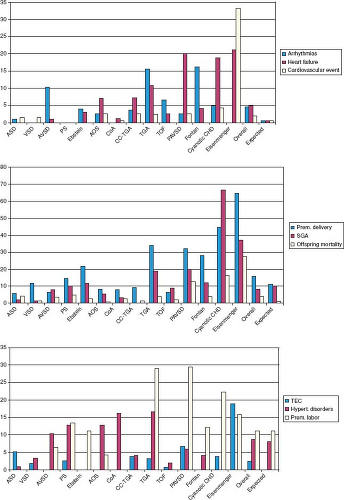
Figure 69.3 shows pooled estimates of maternal and fetal and/or neonatal risks in women with congenital heart disease, stratified
by lesion, derived primarily from retrospective single-center studies. Additional described factors that increase pregnancy risk include the presence of a prosthetic valve or conduit (especially if associated with abnormal prosthetic valve function), occurrence of an obstetric complication such as preeclampsia, and use of anticoagulants or teratogenic drugs. Some of these matters are elaborated further in sections below on prosthetic heart valves, management of anticoagulation and preconception issues. In comprehensive assessment of maternal risk it is helpful to integrate a global risk index with contemporary lesion-specific and other markers of risk, as well as expert opinion. When there is discordance between the global and the lesion-specific estimates of risk, the higher risk estimate should drive the care plan to avoid false reassurance.
by lesion, derived primarily from retrospective single-center studies. Additional described factors that increase pregnancy risk include the presence of a prosthetic valve or conduit (especially if associated with abnormal prosthetic valve function), occurrence of an obstetric complication such as preeclampsia, and use of anticoagulants or teratogenic drugs. Some of these matters are elaborated further in sections below on prosthetic heart valves, management of anticoagulation and preconception issues. In comprehensive assessment of maternal risk it is helpful to integrate a global risk index with contemporary lesion-specific and other markers of risk, as well as expert opinion. When there is discordance between the global and the lesion-specific estimates of risk, the higher risk estimate should drive the care plan to avoid false reassurance.
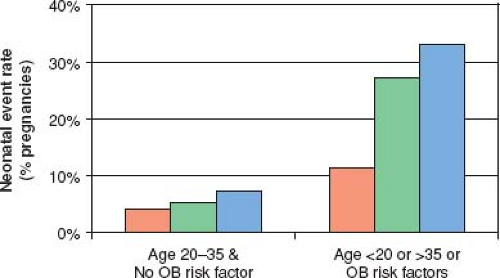
In addition to increased risk of adverse maternal cardiac events, women with cardiac disease are at increased risk for adverse fetal and neonatal complications, which include fetal death, neonatal death, and prematurity with its associated complications (respiratory distress syndrome, intraventricular hemorrhage, small-for-gestational-age birth weight) (27,39). Maternal risk factors for adverse fetal and neonatal outcomes have been identified (see Table 69.2) (32). The risk of neonatal complications is further increased if there are concomitant maternal noncardiac (obstetrical and other) risk factors (see Table 69.2) (Fig. 69.4). Finally, there is cardiac-lesion-specific variation in the risk for adverse obstetric outcomes during pregnancy (see Fig. 69.3) (39).
Women with an intermediate to high risk of adverse maternal cardiac events during pregnancy or those at increased risk for fetal and neonatal complications should be considered for enhanced multidisciplinary surveillance in specialized high-risk cardiac and obstetric programs (29). As well, the impact of maternal heart disease on the probability of adverse obstetric outcomes should be considered when evaluating the need for enhanced intensity of obstetric oversight of pregnancy. The relationship between maternal cardiac status and fetal outcomes may be manifested by changes in uterine and umbilical Doppler flow patterns (40).
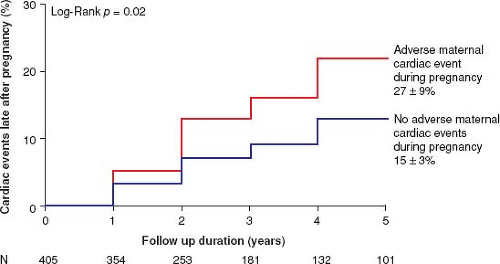
Hemodynamic and hormonal changes of pregnancy may continue to impact maternal outcomes late after pregnancy (41,42,43,44,45). For example, adverse cardiac events late after pregnancy occurred more often in women who had adverse cardiac events during pregnancy (Fig. 69.5) (43). Pregnancy has been associated with an increased likelihood of requiring valve intervention late after pregnancy in women with moderate or severe aortic stenosis (45). At this time, the full extent and mechanisms of the late effects of pregnancy on the heart are poorly understood.
Lesion-Specific Risks and Outcomes
Pooled estimates of the frequency of adverse pregnancy outcomes (maternal cardiac, fetal, neonatal, and obstetric), stratified according to type of congenital heart disease, are presented in Figure 69.3 (39). Pregnancy outcomes stratified solely by diagnosis can be helpful, but in addition it is important to consider the specific surgical history, the history of prior cardiac events, the functional status of the woman and ventricular and valve function, since individual variation in these factors may influence risk over and above the risk imparted by diagnosis alone.
Cardiac Shunts
Women with uncorrected left-to-right shunts, including secundum atrial septal defect (ASD), restrictive ventricular septal defect (VSD), and patent ductus arteriosus (PDA), are at low risk for adverse maternal cardiac events during pregnancy (30,32,46,47,48,49). Potential complications include atrial arrhythmias and heart failure, particularly if the shunt is large. In women with ASD or patent foramen ovale, there is a potential for paradoxical embolization if systemic vasodilation and/or elevation of pulmonary resistance result in transient right-to-left shunting. Atrioventricular septal defects (AVSDs) are more complex lesions, and pregnancy may be less well tolerated. In one study of 62 pregnancies (29 women) in women with AVSD, cardiac complications including persistent NYHA functional class deterioration, arrhythmias and heart failure, were reported to be 23%, 19%, and 2%, respectively (50). If cardiac shunts are associated with pulmonary hypertension, risk is dominated by the impact of the elevated pulmonary vascular resistance, which is discussed elsewhere in this chapter.
Right Ventricular Outflow Tract Obstruction
If pulmonic stenosis is mild or has been previously corrected surgically or by valvuloplasty, it is typically well tolerated during pregnancy (32,46,51). In severe pulmonic stenosis, the increase in preload associated with pregnancy may not be tolerated and may result in atrial arrhythmias or right heart failure. Thus, correction of severe pulmonic stenosis prior to pregnancy should be considered. If decompensation develops during pregnancy, balloon valvuloplasty can be carried out if initial medical therapy proves insufficient (52). Although one group has reported high rates of obstetric and fetal complications in women with pulmonary stenosis (53), this differs from experience reported elsewhere (32,46,51).
Tetralogy of Fallot
Most women with tetralogy of Fallot will have undergone surgical correction with closure of the VSD and relief of right ventricular outflow tract obstruction. After repair, women may be left with pulmonary regurgitation, RV outflow tract obstruction, residual shunts, right ventricular dilation or dysfunction, and atrial or ventricular arrhythmias. In general pregnancy is well tolerated, but risk of complications is increased in the presence of such residua and surgical sequelae. In one series, maternal complications including symptomatic right heart failure, arrhythmias, or both occurred in 12% of pregnancies (54), though other studies have reported lower adverse event rates (55,56,57). Adverse maternal cardiac events have been reported in association with maternal cardiac factors (left ventricular dysfunction, severe pulmonary hypertension, severe pulmonic regurgitation with right ventricular dysfunction or right ventricular outflow tract obstruction) and obstetric risk factors (twin pregnancies) (55,56). Following biventricular repair for double-outlet right ventricle a low risk for maternal cardiac complications was reported in one series of 19 pregnancies; however, fetal and neonatal risks were increased (58).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree