Carole A. Warnes
Pregnancy and Heart Disease
In 2011, guidelines on the prevention of cardiovascular disease in women identified pregnancy complications as risk factors for cardiovascular disease.1 Hypertensive disorders of pregnancy and pregnancy-related diabetes mellitus are independently associated with an increased 10-year cardiovascular risk.2 When encountered in clinical practice, then, pregnancy complications may provide an opportunity for early identification of women at increased risk for development of cardiovascular disease later in life, and perhaps such women should be referred to their primary care physician or a cardiologist to monitor cardiovascular risk factors.3
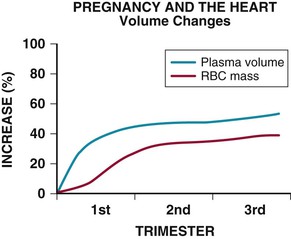
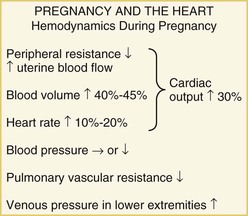
Approximately 2% of pregnancies involve maternal cardiovascular disease, associated with increased risk to both mother and fetus. Most women with cardiovascular disease can have a pregnancy with proper care, but a careful pre-pregnancy evaluation is mandatory. Cardiac disease may sometimes be manifested for the first time in pregnancy because the hemodynamic changes may compromise a limited cardiac reserve.4 Conversely, the symptoms and signs of a normal pregnancy may mimic those of cardiac disease: Lightheadedness, dizziness, shortness of breath, peripheral edema, and even syncope often occur in the course of a normal pregnancy, leading the less wary physician to suspect cardiac disease when none is present. An understanding of the normal findings on cardiac examination in a pregnant patient is therefore important. For those physicians counseling patients with cardiac disease about the potential risks of a pregnancy, a comprehensive knowledge of the underlying defect as well as of the hemodynamic changes that pregnancy will impose is imperative.
With the declining incidence of rheumatic heart disease in Western countries, maternal cardiac disease is now predominantly congenital in origin, although at present the leading causes of maternal death are acquired disease, with myocardial infarction, aortic dissection, and cardiomyopathy recognized as the major clinical syndromes.5
Pre-Pregnancy Counseling
Pre-pregnancy counseling is important to give prospective mothers appropriate information about the advisability of pregnancy and to discuss the risks to her and the fetus. Such patients should be seen in a high-risk pregnancy unit for evaluation including a clinical examination, electrocardiogram, and chest radiograph. An echocardiogram facilitates a detailed evaluation of myocardial function, valvular disease, and pulmonary artery pressures. In patients with congenital heart disease, their perception of normal activity may be skewed, and an exercise test is helpful in delineating their true functional aerobic capacity. In general, patients who cannot achieve more than 70% of their predicted functional aerobic capacity are unlikely to tolerate a pregnancy safely. A careful family history is important to assess whether there is any congenital heart disease not only in the patient’s family but also that of her partner. Genetic counseling also may be offered. A careful discussion of the maternal and fetal risks, and of whether or not these risks might change with time or treatment, is indicated. The possibility that pregnancy might cause irreversible hemodynamic deterioration should be considered, as well as the difficult issue of the long-term outlook for the mother. If the woman is going to pursue a pregnancy, a strategy should be outlined regarding the frequency of follow-up evaluation by the cardiologist, and a plan should be put in place for obstetric and cardiovascular management during the pregnancy as well as during labor and delivery.6
An assessment of maternal cardiac risk may be predicted by the use of a risk index, and several of these have now been published. Worrisome predictors of maternal cardiac events include (1) prior cardiac event (e.g., heart failure, transient ischemic attack, or stroke before pregnancy) or arrhythmia; (2) baseline New York Heart Association (NYHA) class higher than class II or cyanosis; (3) left-sided heart obstruction (mitral valve area smaller than 2 cm2, aortic valve area less than 1.5 cm2, or peak left ventricular outflow tract gradient greater than 30 mm Hg as assessed by echocardiography); and (4) reduced systemic ventricular systolic function (ejection fraction less than 40%).7 More recent publications emphasize the considerable risks when the mother has a mechanical valve prosthesis.8 The use of these risk indices carries limitations, however, in that they are highly population-dependent. Some series, for example, include only patients with congenital heart disease9; others include patients with acquired heart disease, and so-called high-risk patients, such as those with clinically significant pulmonary hypertension or a dilated aorta, may not be identified and are underrepresented. Accordingly, these “predictors” should be used only as a guide, and each case should be considered individually.
During pregnancy, a multidisciplinary team approach is recommended, with close collaboration with the obstetrician, so that the mode, timing, and location of delivery can be planned. The management should be tailored to the specific needs of the patient. During pregnancy, fetal growth is monitored by the obstetric team, and for the woman with congenital heart disease, a fetal cardiac echocardiogram is offered at approximately 22 to 26 weeks of pregnancy to determine whether a congenital cardiac anomaly is present.
Evaluation
Physical Examination
Evaluation of the pregnant patient begins with a thorough physical examination, including cardiac examination (see Chapter 11).
Because of the altered hemodynamics during pregnancy, the physical examination findings in a healthy pregnant woman reflect such changes and may mimic those in cardiac disease. The heart rate increases and the pulse volume often is bounding. By the middle of the second trimester, the jugular venous pressure may be elevated, with brisk descents, because of the volume overload and reduced peripheral resistance. The apical impulse is more prominent, and on auscultation, the first sound may appear loud. Commonly, an ejection systolic murmur can be heard at the left sternal edge, never more than grade 3/6 in intensity, which relates to increased flow through the left or right ventricular outflow tract. A third sound is very common. There should be no diastolic murmur. The second sound also may appear accentuated, and these combined auscultatory features may suggest an atrial septal defect or pulmonary hypertension. Continuous murmurs also may be heard, as either a cervical venous hum or a mammary souffle. Peripheral edema is common as pregnancy advances. If any findings on the physical examination are suggestive of cardiac disease, transthoracic echocardiography should be performed. This investigation facilitates the evaluation of ventricular size and function, valvular heart disease, and any potential shunts (e.g., atrial septal defect, ventricular septal defect) and permits the noninvasive assessment of pulmonary artery pressure.
Laboratory Evaluation
Despite the hemodynamic volume load of pregnancy, most healthy pregnant women have low and stable concentrations of B-type natriuretic peptide (BNP) throughout pregnancy and after delivery. By comparison, women with heart disease have higher BNP levels throughout pregnancy compared with nonpregnant women, and BNP levels below 100 pg/mL may have a good negative predictive value for predicting adverse cardiac events.11
Imaging
Presented next is an overview of some important considerations with various imaging studies in the pregnant patient (see Chapters 14 to 18 for additional details of these modalities).
Chest Radiography
A chest radiograph is not obtained routinely in any pregnant patient because of concern about radiation exposure to the fetus, but it should be considered when the history and clinical findings raise concerns about maternal cardiac status and in cases of new-onset dyspnea or heart failure. The chest radiograph in a normal healthy patient may show slight prominence of the pulmonary artery, and as pregnancy advances, elevation of the diaphragm may suggest an increase in the cardiothoracic ratio.
Transthoracic Echocardiography
Transthoracic echocardiography is the cornerstone of cardiac evaluation in pregnancy and facilitates differentiation of the features of cardiac disease from those of a normal pregnancy. This imaging study most frequently is used to determine the ventricular function, to assess the status of native and prosthetic valve disease, and, by determination of the tricuspid regurgitant velocity, to assess pulmonary artery pressure. For those patients with congenital heart disease, a detailed assessment of any shunt and complex anatomy may be made.
During pregnancy, because of the increased cardiac output, the velocities across the left and right ventricular outflow tracts increase, which may mimic an increase in outflow tract gradient. Careful comparison of the two-dimensional anatomic appearances will help differentiate this from a true valvular abnormality, and calculation of valve area will be helpful. Similarly, because of the increased stroke volume, any valvular regurgitation will appear to be accentuated. Serial echocardiograms may be particularly useful in a patient with a mechanical valve prosthesis, who is vulnerable to development of thrombosis during pregnancy. The valve area calculation may be more helpful than a simple measurement of valve gradient; the latter may appear to be increased as pregnancy advances because the circulation becomes more hyperkinetic and cardiac output increases.
In patients with impaired ventricular function, particularly those with cardiomyopathy, echocardiography plays the most important role in assessing left ventricular function. In a normal pregnancy, the left ventricular end-diastolic measurement is increased, and there may be similar increases in right ventricular size as well as in the volumes of both atria. Measurement of ejection fraction is determined by changes in preload and afterload, and with the patient in the supine position, preload may be reduced because the fetus may compress the inferior vena cava.
Transesophageal Echocardiography
Transesophageal echocardiography is seldom performed during pregnancy but may be necessary to provide more detailed imaging of valvular disease or to determine the presence or absence of a shunt or intracardiac thrombus. In addition, it may be useful to confirm or rule out the presence of endocarditis, to facilitate detection of a valvular vegetation or perivalvular abscess. Transesophageal echocardiography can be performed safely, although careful monitoring of maternal oxygen saturation is necessary if midazolam is used for sedation.
Fetal Echocardiography
Excellent imaging of the fetal heart usually can be achieved by 20 weeks’ gestation. The four-chamber view may be obtained in most pregnancies and should demonstrate two atrioventricular valves and the crux of the heart and will determine whether two ventricles of equal size are present. The patent foramen ovale also should be demonstrated. Typically, the heart should be smaller than one-third of the size of the fetal thorax.
Magnetic Resonance Imaging and Computed Tomography
Limited data are available for magnetic resonance imaging (MRI) in pregnancy, but it probably is safe, especially after the first trimester. Gadolinium should be avoided. Because of the risks of radiation exposure to the fetus, computed tomography (CT) is not recommended unless absolutely necessary.
Management during Pregnancy
Medical Therapy
Patients who are otherwise healthy may require little or no specific treatment other than the usual obstetric recommendations and monitoring. Patients with NYHA class I or II status may need to limit strenuous exercise and should have adequate rest, supplementation of iron and vitamins to minimize the anemia of pregnancy, low-salt diet if the possibility of ventricular dysfunction is a concern, and require regular cardiac and obstetric evaluations, the frequency of which must be individualized. Patients in NYHA class III or IV may need hospital admission for bed rest and close monitoring and may require early delivery if maternal hemodynamic compromise is present.
Surgical Management
Cardiac surgery during pregnancy is seldom necessary and should be avoided whenever possible. A higher risk of fetal malformation and loss has been documented when cardiopulmonary bypass is performed in the first trimester; if it is performed in the last trimester, the likelihood of precipitating premature labor is greater. The “optimal time” for such intervention appears to be between 20 and 28 weeks of gestation, and the fetal outcome may be improved by use of normothermic rather than hypothermic extracorporeal circulation, higher pump flows, higher pressures (mean blood pressure of 60 mm Hg), and as short a bypass time as possible. Obstetric monitoring of the fetus during the procedure is recommended so that fetal bradycardia may be dealt with promptly and uterine contractions may be controlled.
Early reports suggested that maternal mortality associated with cardiopulmonary bypass during pregnancy occurred at a rate of 3% to 15%. In the current era, however, with the use of the interventions just outlined, cardiothoracic surgery can be performed with relative safety during pregnancy, with a maternal mortality rate similar to that in the nonpregnant state unless the surgery is emergent. Maternal functional class is an important predictive factor for maternal death. Fetal complications (prematurity and death) are associated with urgent high-risk surgery, maternal comorbidity, and early gestational age.12 A multidisciplinary approach is preferable to optimize the outcome for both mother and baby.
High-Risk Pregnancies
In some situations, the maternal risk from pregnancy is very high, and the patient should be counseled to avoid pregnancy and sometimes even to consider termination of pregnancy if it occurs (Table 78-1). No data exist regarding the precise level of pulmonary hypertension that poses a major threat to the mother, but in my experience, systolic pulmonary artery pressures higher than 60% to 70% of the systemic pressure are likely to be associated with maternal compromise; in these circumstances, pregnancy is best avoided. Women who have a left ventricular ejection fraction less than 40% from any cause are not likely to withstand the volume load that pregnancy imposes and should be advised not to become pregnant. Because pregnancy is associated with a decrease in peripheral resistance, symptomatic patients with significant stenotic cardiac lesions (see Table 78-1) are likely to deteriorate during pregnancy. Patients with a dilated aortic root more than 45 mm in diameter are vulnerable to progressive aortic dilation, dissection, and rupture during pregnancy, particularly those patients with Marfan syndrome. This occurs not only because of the increased stroke volume but probably also because the gestational hormonal changes may be additive to the underlying histologic abnormality in the aortic media.
Cardiovascular Diseases
Congenital Heart Disease
Today, maternal cardiac disease in Western societies is mostly congenital in origin. This predominance relates both to the frequency of congenital heart disease (almost 1 in 100 babies; see Chapter 62) and to the advances in reparative cardiac surgery during the last 50 years. Some patients will present for the first time in pregnancy with symptoms and learn that they have congenital heart disease. Others with repaired defects may encounter cardiac problems during pregnancy, the most common being heart failure and arrhythmias. All patients, whether or not they have had cardiac repair, should receive a detailed evaluation and appropriate counseling before pregnancy is considered.
Atrial Septal Defect
Secundum atrial septal defect is one of the most common congenital heart defects. Patients with even a large secundum atrial septal defect usually tolerate pregnancy without complications unless concomitant pulmonary hypertension or atrial fibrillation is present. The volume load on the right ventricle usually is well tolerated. Meticulous attention should be paid to the maternal leg veins, particularly during and after delivery, because deep vein thrombosis could precipitate a paradoxical embolus and stroke. Elective closure of an atrial septal defect by device or operative repair is preferable before pregnancy is contemplated.
Ventricular Septal Defect
Patients with small ventricular septal defects usually tolerate pregnancy without difficulty. In the setting of a large ventricular septal defect and pulmonary hypertension, patients should be counseled not to proceed with a pregnancy (see later discussion of Eisenmenger syndrome).
Patent Ductus Arteriosus
In patients with patent ductus arteriosus, small ducts with normal or near-normal pressures usually cause no hemodynamic perturbations during pregnancy. With a large shunt, the added volume load of pregnancy may potentially precipitate left ventricular failure. Patients with pulmonary hypertension should be counseled that pregnancy is contraindicated.
Congenital Aortic Stenosis
Aortic stenosis in women of childbearing age usually is secondary to a bicuspid aortic valve. A detailed two-dimensional anatomic and Doppler echocardiographic assessment of the valve function should be performed before pregnancy is contemplated. In addition, a careful examination of the entire thoracic aorta is indicated to look for associated aortopathy; even with a functionally normal valve, an aortic dilation or ascending aortic aneurysm may be present. Pregnancy usually is considered to be contraindicated if the aortic dimension is larger than 4.5 cm, and surgical repair should be considered if the aorta is larger than 5 cm (27.5 mm/m2) (Fig. 78-3).
Mild aortic stenosis usually is well tolerated, provided that the patient has a normal exercise capacity and no symptoms.13 Moderate stenosis is sometimes well tolerated, but the patient needs to be evaluated carefully before pregnancy. In the absence of symptoms, with a normal result on exercise testing without ST-T wave changes, pregnancy in a compliant patient monitored with careful management is likely to be successful. With severe aortic stenosis (valve area smaller than 1 cm2) or a mean gradient greater than 50 mm Hg, the patient should be counseled not to have a pregnancy. The decrease in peripheral resistance during pregnancy will exaggerate the aortic gradient, which may precipitate symptoms. Patients may respond to bed rest and the administration of beta blockers, but an early delivery may be necessary.
The risk to the mother of continuing the pregnancy versus delivery of the baby early by cesarean section needs to be considered. Labor and delivery can be particularly problematic in such patients because of the abrupt hemodynamic changes, particularly the abrupt fall in afterload when the baby is delivered. Blood loss at the time of parturition also can precipitate maternal collapse. Epidural analgesia needs to be carefully and slowly administered, and spinal block should be avoided because of the potential for hypotension. Delivery may be facilitated by central venous pressure monitoring or the use of a Swan-Ganz catheter to maintain optimum hemodynamics, which should be continued for at least 24 hours after delivery. One study of 35 women with aortic stenosis and 58 pregnancies (53 successful) reported cardiac complications in 9.4%, with both obstetric (22.6%) and perinatal (24.5%) complications being found more frequently than normal.14 Furthermore, 7 premature births (13.2%) and 7 small-for-gestational-age births (13.2%) were encountered. Pregnancy in women with severe aortic stenosis was characterized by an increased incidence of heart failure and premature labor and shorter pregnancy duration. In addition, women with moderate or severe aortic stenosis who are symptomatic during pregnancy are at greater likelihood of requiring cardiac interventions.15
Several small reports have reviewed percutaneous aortic balloon valvuloplasty during pregnancy; this can be accomplished safely, provided that the valve anatomy is favorable and the procedure is performed by an experienced interventionalist. Radiation exposure to the fetus can be minimized by lead screening of the mother’s abdomen and pelvis. The procedure should be performed in centers with extensive experience and surgical back-up; if it is undertaken after 26 weeks of pregnancy, obstetric standby should be available in case of premature labor.
Coarctation of the Aorta
Women with coarctation of the aorta may present with symptoms for the first time during pregnancy, typically systemic hypertension. A significant coarctation impairs flow to both the uterus and fetus, which may result in small-for-dates babies or even fetal loss. Therapeutic options include antihypertensive therapies, percutaneous stenting of the coarctation, and surgical intervention (see Chapters 57 and 62). Aggressive antihypertensive therapy should be avoided because of the chance of placental hypoperfusion. Because of the associated aortopathy, the entire aorta is vulnerable to dilation, aneurysm, and dissection. When the presence of a coarctation is known, the entire aorta should be imaged at the time of pre-pregnancy counseling. Most women, however, will have a successful pregnancy with proper care.
Pulmonary Stenosis
Pulmonary stenosis usually is well tolerated during pregnancy, particularly if the right ventricular pressure is less than 70% of systemic pressure and sinus rhythm is maintained. If necessary, balloon pulmonary valvuloplasty can be performed, with shielding of the fetus from radiation.
Cyanotic Heart Disease
Cyanosis poses risks for both mother and fetus.16 The decrease in peripheral resistance that accompanies pregnancy augments the right-to-left shunt and may exaggerate the maternal cyanosis. Because of the erythrocytosis that accompanies cyanosis and the propensity to thrombosis, women in whom venous thrombosis develops are at risk of paradoxical embolus and stroke. Maternal hypoxia imposes a pronounced handicap on fetal growth and survival. Presbitero and colleagues17 evaluated 44 women with 96 pregnancies (excluding patients with Eisenmenger syndrome) and confirmed that the degree of maternal cyanosis has a profound impact on fetal outcome. When the maternal oxygen saturation is less than 85%, the fetal outcome is poor, with only 2 of 17 pregnancies (12%) resulting in live-born infants (Table 78-2). Conversely, when the maternal oxygen saturation is 90% or higher, 92% of the pregnancies result in a live birth. Maternal cardiovascular complications occurred in 14 patients (32%). Eight patients had heart failure, and bacterial endocarditis occurred in two patients, both with surgically palliated tetralogy of Fallot. Two patients had thrombotic complications, one pulmonary and one cerebral.
TABLE 78-2
Fetal Outcome in Cyanotic Congenital Heart Disease and Its Relationship with Maternal Cyanosis
| NO. OF PREGNANCIES | NO. OF LIVE BIRTHS | % BORN ALIVE | |
| Hemoglobin (g/dL)* | |||
| ≤16 | 28 | 20 | 71 |
| 17-19 | 40 | 18 | 45 |
| ≥20 | 26 | 2 | 8 |
| Arterial oxygen saturation (%)† | |||
| ≤85 | 17 | 2 | 12 |
| 85-89 | 22 | 10 | 45 |
| ≥90 | 13 | 12 | 92 |

* Hemoglobin concentration unknown in two pregnancies.
† Arterial oxygen saturation unknown in 44 pregnancies.
From Presbitero P, Somerville J, Stone S, et al: Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation 89:2673, 1994.
In addition to the degree of maternal cyanosis, right ventricular function must be assessed before pregnancy by echocardiography or MRI. The type of maternal cardiac lesion present also will affect the propensity of the baby to inherit congenital cardiac disease. For those women with conotruncal abnormalities (tetralogy or pulmonary atresia), screening for 22q11 deletion is recommended, because this has autosomal dominant transmission, and the offspring have a 50% chance of inheriting the genetic defect.
Ebstein’s Anomaly
The safety of a pregnancy in patients with Ebstein’s anomaly depends on right ventricular size and function, degree of tricuspid regurgitation, and presence or absence of an atrial communication. The last is present in approximately 50% of the cases, and if the patient is cyanotic at rest, the risk of pregnancy increases considerably. An atrial communication poses the added potential risk of a stroke from a paradoxical embolus, and meticulous attention should be paid to the possibility of maternal deep vein thrombosis. Atrial arrhythmias may not be well tolerated in the pregnant woman with this anomaly, and both atrial fibrillation and reentry tachycardia are common. Accessory bypass tracts causing preexcitation may precipitate rapid tachycardia, which add to the burden of a poorly functioning right ventricle. After successful surgical repair or replacement of the tricuspid valve, pregnancy may be well tolerated.
Congenitally Corrected Transposition (L-Transposition)
The L-transposition anomaly is characterized by atrioventricular discordance and ventriculoarterial discordance; thus the systemic ventricle is the morphologic right ventricle. Patients may have a successful pregnancy so long as the ejection fraction of the systemic ventricle is preserved and no significant associated anomalies are present. The most common of these is systemic atrioventricular valve (tricuspid) regurgitation, which contributes to systemic ventricular dysfunction. Other lesions, such as ventricular septal defect, pulmonary stenosis, and complete heart block, may coexist and compromise the patient’s ability to have a successful pregnancy.
Repaired Congenital Heart Disease
Very few operations for congenital heart disease can be considered curative, and almost all patients have residua and sequelae that must be carefully evaluated at the time of pre-pregnancy counseling. (See Chapter 62 for a detailed discussion of this topic.)
Tetralogy of Fallot
Most women with tetralogy of Fallot will have had previous surgical repair and should be free of cyanosis. An occasional adult will be seen who has not had previous surgery or in whom palliation was achieved with a surgically created shunt (e.g., Blalock-Taussig). In such cases, pregnancy may pose a risk, depending on the degree of cyanosis, as noted earlier. The fall in peripheral resistance augments the right-to-left shunt through the ventricular septal defect, causing worsening cyanosis, with risk to both mother and fetus.
For those patients with previous definitive surgical repair, a careful assessment of any hemodynamic residua and sequelae should be undertaken before advice is given about the safety of a pregnancy. The clinical and echocardiographic evaluation should focus on the presence of lesions, such as residual pulmonary regurgitation, which is common after repair, and associated right ventricular dysfunction and tricuspid regurgitation. The volume load of pregnancy may not be well tolerated in these circumstances, and superimposed atrial and even ventricular arrhythmias may add to the hemodynamic stresses. Additional “volume lesions,” such as ventricular septal defects and aortic regurgitation, as well as residual right ventricular outflow tract obstruction, should be evaluated. For those women with an effective surgical repair, good exercise capacity, and minimal residua, pregnancy may be well tolerated, provided that they are properly managed.18 Genetic counseling should be offered to look for 22q11 deletion. In the absence of a parental chromosomal abnormality and a family history of other congenital cardiac disease, the risk of the fetus having a congenital cardiac anomaly is approximately 5% to 6%, similar to the risk of inheritance of many congenital cardiac lesions.
Transposition of the Great Arteries (D-Transposition)
All patients with transposition of the great arteries (D-transposition) will have had surgery in childhood, commonly an atrial baffle procedure (Mustard or Senning operation), which leaves the morphologic right ventricle as the systemic pump. Function of the systemic ventricle should be assessed clinically and echocardiographically before pregnancy, as well as the degree of tricuspid (systemic) atrioventricular valve regurgitation and degree of baffle obstruction, the residual atrial septal defect, and the presence or absence of atrial arrhythmias, which are common complications. Dysfunction of the systemic ventricle may be a contraindication to pregnancy. In the more recent surgical era, patients are more likely to have had an arterial switch procedure. Residua include aortic and pulmonary regurgitation as well as stenosis of the translocated coronary arteries. These hemodynamics should all be evaluated at the time of pre-pregnancy counseling.
Coarctation
The evaluation of the woman with repaired coarctation should include an assessment of the coarctation repair site to exclude residual or recurrent coarctation or aneurysm formation and an imaging study to assess the entire aorta to rule out dilation or aneurysm formation, which is most common in the ascending aorta. The aortic valve and left ventricular function also should be assessed. The outcome for both mother and baby usually is favorable. For patients with mild dilation of the aorta, vaginal delivery with a short second stage is reasonable, but in those with evidence of aortic instability, a cesarean section is preferable.
Univentricular Heart and Fontan Operations
Women who have undergone univentricular heart and Fontan operations are at increased risk for maternal complications, particularly atrial arrhythmias, which may cause profound hemodynamic deterioration. They are particularly vulnerable to development of thrombosis in the Fontan circuit because of the sluggish flow and prothrombotic state of pregnancy. Function of the single ventricle may deteriorate as a consequence of the volume load of pregnancy, and the risk of miscarriage also appears to be significantly increased.19
Pulmonary Hypertension
Pulmonary hypertension (see Chapter 74
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree