The long-term event-free survival (EFS) after acute myocardial infarction (AMI) is largely uninvestigated. We analyzed noninvasive clinical variables in association with long-term EFS after AMI. The present prospective study included 504 consecutive patients with AMI at 3 hospitals from 1995 to 1998 (Adria, Bassano, Conegliano, and Padova Hospitals [ABC] study). Thirty-seven variables were examined, including demographics, cardiovascular risk factors, in-hospital characteristics, and blood components. The end point was 10-year EFS. Logistic and Cox regression models were used to identify the predictive factors. We compared 3 predictive models according to the goodness of fit and C-statistic analyses. At enrollment, the median age was 67 years (interquartile range 58 to 75), 29% were women, 38% had Killip class >1, and the median left ventricular ejection fraction was 51% (interquartile range 43% to 60%). The 10-year EFS rate was 19%. Both logistic and Cox analyses identified independent predictors, including young age (hazard ratio 1.2, 95% confidence interval 1.1 to 1.3, p = 0.0006), no history of angina (hazard ratio 1.4, 95% confidence interval 1.1 to 1.8, p = 0.009), no previous myocardial infarction (hazard ratio 1.4, 95% confidence interval 1.1 to 1.7, p = 0.01), high estimated glomerular filtration rate (hazard ratio 0.8, 95% confidence interval 0.7 to 0.9, p = 0.001), low albumin/creatinine excretion ratio (hazard ratio 1.2, 95% confidence interval 1.1 to 1.3, p <0.0001), and high left ventricular ejection fraction (hazard ratio 0.8, 95% confidence interval 0.7 to 0.9, p = 0.006). These variables had greater predictive power and improved the predictive power of 2 other models, including Framingham cardiovascular risk factors and the recognized predictors of acute heart damage. In conclusion, 10-year EFS was strongly associated with 4 factors (ABC model) typically neglected in studies of AMI survival, including estimated glomerular filtration rate, albumin/creatinine excretion ratio, a history of angina, and previous myocardial infarction. This model had greater predictive power and improved the power of 2 other models using traditional cardiovascular risk factors and indicators of heart damage during AMI.
Fatal and nonfatal adverse events are common in the short and long term after acute myocardial infarction (AMI). It is currently considered that much of the increased cardiovascular (CV) risk remains unexplained. To improve the development of new therapies, we require a better understanding of the natural history of coronary artery disease. Many studies have investigated the prognosis after AMI, but few have focused on the factors associated with event-free survival (EFS), particularly in the long term. Furthermore, EFS can be considered the clinical equivalent to no progression of coronary artery disease. The aim of the present study was to investigate and compare how the major CV risk factors and a number of noninvasive clinical variables are associated with EFS in a 10-year follow-up study after AMI.
Methods
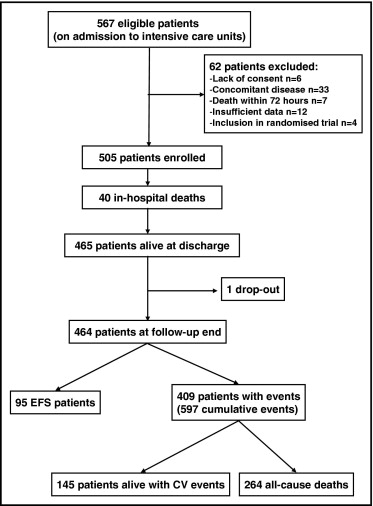
The Adria, Bassano, Conegliano, and Padova Hospital Study (ABC Study) is an ongoing, prospective investigation designed to reflect, as closely as possible, an unbiased population of patients with AMI. It included 567 consecutive, white patients admitted with definite AMI to the intensive care units of the Adria, Bassano, and Conegliano Hospitals (in northeast Italy) from June 21, 1995 to January 19, 1998. The original aim of the ABC study was to follow the natural long-term history of a sample of unselected patients with AMI and to evaluate the prognostic value of a number of baseline clinical variables. The criteria used for AMI diagnosis have been previously reported. The patients were excluded when they displayed chronic renal failure, defined as a documented history of an estimated glomerular filtration rate (eGFR) <1.0 mL/s/1.73 m 2 (conventional units <60 mL/min/1.73 m 2 ) for 3 months, with or without kidney damage (n = 3). They were also excluded for nephrotic proteinuria (n = 2), dialysis treatment (n = 1), concomitant acute infection (n = 15), myocardial reinfarction within 3 days of admission (n = 3), surgical treatment of bone fractures (n = 2), recent surgery (n = 2), menstrual flow (n = 1), neoplastic disease (n = 4), death within 3 days of admission (n = 7), or insufficient data (n = 12). These conditions could potentially affect the variables investigated in the present study. Ten additional patients were excluded because of a lack of consent or because they were involved in a randomized treatment trial. Thus, the follow-up included 505 patients ( Figure 1 ). All enrolled patients provided written informed consent, and the hospital ethics committees approved the study.
At enrollment, we collected a thorough patient history from the medical records and patient interviews. Unless otherwise indicated, all baseline clinical and laboratory data reported in the present study were obtained during the first 3 days of hospitalization in the intensive coronary care unit. On admission and every 4 hours thereafter, the serum enzyme levels and 12-lead electrocardiograms were obtained. Venous blood was drawn for biochemical determinations. The blood pressure and heart rate were measured between 7 and 8 a.m. , and the mean of 3 recordings was used in the analyses. The presence and degree of heart failure, assessed according to the Killip classification, the presence of atrial fibrillation/flutter, ventricular tachy- and bradyarrhythmias were recorded during the first week after enrollment. The left ventricular ejection fraction (LVEF) was assessed using 2-dimensional echocardiography according to Simpson’s method. The LVEF was missing for 103 patients who underwent echocardiography after discharge from the intensive care unit or had technically inadequate echocardiographic images. The records were examined by 2 physicians with no knowledge of patient clinical data. The eGFR at baseline was calculated using the modified Modification of Diet in Renal Disease equation. Albumin excretion was measured with 24-hour urine collection samples by radioimmunoassay and expressed as the albumin/creatinine ratio. Standard urinalysis was performed at urinary sample collection.
At 1, 3, 5, 7, and 10 years after recruitment, each patient was telephoned for a clinical checkup. The prespecified primary end point of the present study was 10 years free from death and major coronary events or stroke. An event was defined as any of the following: death from any cause, nonfatal reinfarction or stroke, angina at rest with electrocardiographic changes and/or congestive heart failure requiring hospitalization, revascularization (coronary artery bypass grafting or nonprimary percutaneous coronary angioplasty), and heart transplantation. When revascularization procedures occurred during AMI or unstable angina, it was recorded as a single event (e.g., AMI treated with primary percutaneous coronary angioplasty was recorded as AMI). The absence of any of these features was considered EFS. The 2 prespecified secondary end points were EFS after discharge (i.e., excluding in-hospital mortality) and a subanalysis of only CV events (i.e., excluding non-CV death, when it was the first event). All data regarding the events were obtained from the scheduled checkup records, public administration and hospital records, family doctors, and death certificates. In keeping with the procedure used in the Global Registry of Acute Coronary Events (GRACE) study, events occurring before enrollment were entered into the Cox regression models as explanatory variables. The reports were also obtained regarding changes in the major CV risk factors (i.e., smoking, hypertension, hypercholesterolemia, diabetes mellitus, and physical activity) and medications during follow-up. The prehospital time delay was defined as the interval from the onset of symptoms to arrival at the coronary care unit. Hypertension was defined as a documented history of hypertension by administration of antihypertensive therapy or a doctor’s report of blood pressure ≥140/90 mm Hg. Hypercholesterolemia was defined as having a total cholesterol level of ≥6.2 mmol/L and/or treatment with lipid-lowering medication. Physical activity was considered ≥3 sessions of isotonic activities weekly that lasted ≥40 minutes.
The measured variables were analyzed as both continuous and quartiles of increasing value. Log transformations were used to correct for positive-skewed distributions, as appropriate. The unpaired Student t test and the Pearson chi-square test was used for the measured and categorical variables, respectively. Both logistic and Cox proportional hazard regression models were used to describe the influence of variables on EFS during follow-up. Logistic regression analyses were fit to the presence/absence of events. Cox regression models were fit to the intervals elapsed before an event. Scaled Schoenfeld residuals were used to test the proportionality assumption. The proportional hazards assumption of the Cox model was violated for several variables (p <0.05) because of early events. The typical effect of this violation was that statistical comparisons were more conservative, and the 95% confidence limits were wider for the hazard ratios. After censoring in-hospital mortality cases, the proportional hazards assumption was verified for all variables (p >0.30). The variables were first tested at the univariate level and after age and gender adjustment. All multivariate analyses used logistic and Cox regression models with backward elimination. To avoid exclusion of potentially significant predictors, once the final model was obtained, each of the excluded variables was retested in the model. Estimated coefficients and standard errors are reported for both logistic and Cox regression models. The risk estimate was quantified as the odds ratio for logistic regression analysis and as the hazard ratio for the Cox regression analyses, with 95% confidence intervals. The interval ranged from the first day of hospital admission to the first nonfatal or fatal event or to the censored time.
Only variables that were significant on both logistic and Cox multivariate analyses were included in the ABC model. The ABC model’s predictive power was compared with 2 other prespecified models: (1) Framingham CV risk factors (i.e., current smoking, physical activity, hypertension, hypercholesterolemia, and diabetes mellitus), termed the “CV risk factor model”; and (2) well-recognized clinical variables associated with acute heart damage (i.e., prehospital time delay, creatine kinase-MB peak, Killip class, Q-/non–Q-wave AMI, and atrial fibrillation), termed the “acute-heart model.” The improvement in predictive power was tested with the goodness-of-fit test according to the likelihood ratio chi-square analysis. Model discrimination was measured using the area under the receiver operating characteristic curve, also called the C-statistic. The Hosmer-Lemeshow test was used to measure model calibration.
The baseline characteristics were summarized as the median and interquartile range for the continuous variables and numbers and percentages for categorical variables. The variables were tested for collinearity before evaluation in the regression models. When 2 variables (e.g., creatine kinase peak and creatine kinase-MB peak) were highly correlated (r ≥0.7), we eliminated the less significant variable or the variable believed to be less clinically important. To detect whether an association between a variable and outcome produced a J or U shape, all variables were checked for conformity to increasing (or decreasing) gradients. The variables significantly associated with the outcomes in the multivariate models were tested for interactions. Unless otherwise indicated, 2-tailed p values <0.05 were deemed significant. Statistical analyses were performed using SYSTAT, version 13 (Systat Software, Chicago, Illinois) and JMP, version 4.0, for Windows 2000 (SAS Institute, Cary, North Carolina).
Results
During follow-up, 1 patient withdrew consent, and the data were censored at that time. Thus, 504 patients had 10-year follow-up data or had died and that data were used in the logistic and Cox analyses. This represented a total of 3,271.3 person-years of follow-up. At the end of the follow-up period, 409 patients had had 1 to 5 events, for a total of 597 cumulative events. Of the 504 patients, 95 had achieved EFS ( Figure 1 ). The event rate was 18.25 events/year of follow-up for each 100 patients. The median interval to the first event was 22.5 months (interquartile range 4.0 to 94.0). Figure 2 lists the main causes of events. The differences between the patients with and without events after AMI are listed in Table 1 .
| Variable | Overall Population (n = 504) | Patients Free of Events (n = 95) | Patients With events (n = 409) | p Value |
|---|---|---|---|---|
| Age (years) | 67 (58–75) | 59 (53–66) | 70 (62–77) | <0.0001 |
| Women | 29% | 20% | 31% | 0.04 |
| Education (high school or more) | 25% | 30% | 24% | 0.18 |
| Current smoker | 38% | 55% | 34% | <0.0001 |
| Physical activity | 6% | 8% | 5% | 0.25 |
| Hypertension | 47% | 33% | 50% | 0.002 |
| Hypercholesterolemia | 24% | 24% | 24% | 0.96 |
| Diabetes mellitus | 24% | 13% | 27% | 0.004 |
| Body mass index (kg/m 2 ) | 26 (24–28) | 26 (24–28) | 26 (24–28) | 0.98 |
| Alcohol use | 74% | 76% | 74% | 0.66 |
| Coffee use | 87% | 93% | 86% | 0.07 |
| Family coronary heart disease | 24% | 23% | 24% | 0.79 |
| Angina pectoris | 20% | 6% | 23% | <0.0001 |
| Previous myocardial infarction | 21% | 7% | 25% | <0.0001 |
| In-hospital characteristics | ||||
| Prehospital time delay (min) ⁎ | 185 (120–535) | 175 (125–292) | 235 (115–590) | 0.002 |
| Systolic blood pressure (mm Hg) | 119 (107–130) | 116 (105–128) | 122 (112–132) | 0.08 |
| Diastolic blood pressure (mm Hg) | 76 (69–82) | 74 (68–79) | 77 (71–82) | 0.43 |
| Heart rate (beats/min) | 70 (63–80) | 68 (61–75) | 71 (64–82) | 0.001 |
| Anterior myocardial infarction | 33% | 37% | 32% | 0.39 |
| Q-wave myocardial infarction | 74% | 84% | 72% | 0.01 |
| Creatine kinase peak (U/L) ⁎ | 1,077 (587–1,959) | 1,286 (694–2,296) | 1,067 (554–1,866) | 0.03 |
| Creatine kinase-MB peak (U/L) ⁎ | 126 (69–236) | 162 (75–274) | 118 (66–229) | 0.13 |
| Killip class >1 † | 38% | 19% | 43% | <0.0001 |
| Tachyarrhythmias † ‡ | 24% | 24% | 23% | 0.87 |
| Bradyarrhythmias † ‡ | 8% | 7% | 9% | 0.70 |
| Atrial fibrillation/flutter † | 13% | 3% | 15% | 0.002 |
| Left ventricular ejection fraction (%) (n = 401) | 51 (43–60) | 58 (50–64) | 50 (42–60) | <0.0001 |
| Blood components | ||||
| Hemoglobin (g/L) | 137 (126–147) | 139 (132–148) | 136 (125–147) | 0.98 |
| Blood glucose (mmol/L) | 6.9 (5.8–9.5) | 6.6 (5.7–8.4) | 7.0 (5.8–9.9) | 0.02 |
| Total cholesterol (mmol/L) | 5.4 (4.6–6.2) | 5.4 (4.7–6.2) | 5.3 (4.5–6.3) | 0.15 |
| High-density lipoprotein cholesterol (mmol/L) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 0.47 |
| Triglycerides (mmol/L) | 1.4 (1.0–2.0) | 1.4 (1.1–2.0) | 1.4 (1.0–2.0) | 0.85 |
| Potassium (mmol/L) | 4.1 (3.8–4.4) | 4.1 (3.8–4.4) | 4.1 (3.8–4.4) | 0.11 |
| Uric acid (μmol/L) | 339 (273–404) | 309 (243–374) | 339 (279–410) | 0.13 |
| Kidney and endothelial function | ||||
| Plasma creatinine (μmol/L) | 88 (80–106) | 80 (71–88) | 88 (80–106) | <0.0001 |
| Estimated glomerular filtration rate (mL/s ×1.73 m 2 ) ⁎ | 1.2 (0.9–1.4) | 1.3 (1.2–1.6) | 1.1 (0.9–1.4) | <0.0001 |
| Albumin/creatinine excretion ratio (mg/mmol) ⁎ | 0.8 (0.3–2.6) | 0.3 (0.2–0.9) | 1.0 (0.4–3.6) | <0.0001 |
| In-hospital and follow-up medications | ||||
| Thrombolysis | 40% | 57% | 36% | <0.0001 |
| Adrenergic agent | 10% | 2% | 11% | 0.005 |
| β-Receptor blocker | 43% | 59% | 40% | <0.0001 |
| Calcium channel blocker | 44% | 49% | 43% | 0.32 |
| Nitrate | 76% | 69% | 78% | 0.06 |
| Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker | 64% | 57% | 65% | 0.14 |
| Diuretics | 51% | 31% | 55% | <0.0001 |
| Antiplatelets | 82% | 95% | 80% | <0.0001 |
| Anticoagulants | 17% | 6% | 19% | 0.002 |
| Digitalis | 22% | 5% | 26% | <0.0001 |
| Antiarrhythmics | 13% | 9% | 14% | 0.23 |
| Lipid-lowering drug | 34% | 45% | 31% | 0.01 |
| Cardiovascular risk factor modification during follow-up | ||||
| Current smoker | 12% | 9% | 24% | <0.0001 |
| Physical activity | 23% | 43% | 18% | <0.0001 |
| Hypertension | 58% | 49% | 60% | 0.07 |
| Hypercholesterolemia | 40% | 52% | 37% | 0.01 |
| Diabetes mellitus | 29% | 18% | 31% | 0.008 |
⁎ p Values were calculated using Log-transformed data.
† During first 7 days of hospital stay.
‡ Tachyarrhythmia and bradyarrhythmia, excluding perithrombolytic period.
At the univariate level, young age was strongly associated, and gender was not associated, with EFS ( Table 2 ). Of the 37 baseline variables tested, 17 were associated with EFS at the univariate level. Both the logistic and Cox analyses showed that the following were associated with EFS, independent of age and gender: no diabetes mellitus, no history of angina and/or myocardial infarction, low Killip class, high LVEF, no atrial fibrillation/flutter, high eGFR, and low albumin/creatinine ratio ( Table 2 ). Only the Cox regression analysis showed associations between EFS and heart rate, blood glucose, triglycerides, and uric acid ( Table 2 ). Among the CV risk factors that had changed during follow-up, the absence of diabetes mellitus and physical activity tended to be associated with EFS ( Table 2 ).
| Variable | Univariate Logistic Regression | Age- and Gender-Adjusted Logistic Regression | Age- and Gender-Adjusted Cox Regression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β ± SE | OR (95% CI) | p Value | β ± SE | OR (95% CI) | p Value | β ± SE | HR (95% CI) | p Value | |
| Young age | 0.83 ± 0.12 | 2.3 (1.8–2.9) | <0.0001 | — | 0.35 ± 0.05 | 1.4 (1.3–1.5) | <0.0001 ⁎ | ||
| Male gender | 0.51 ± 0.27 | 1.7 (1.1–2.8) | 0.06 | −0.23 ± 0.31 | 0.8 (0.4–1.4) | 0.45 † | 0.07 ± 0.11 | 1.1 (0.8–1.3) | 0.55 † |
| Low school education | −0.38 ± 0.25 | 0.7 (0.4–1.1) | 0.13 | 0.06 ± 0.23 | 1.1 (0.6–1.8) | 0.82 | 0.01 ± 0.12 | 1.0 (0.8–1.3) | 0.92 |
| No/current smoking | −0.86 ± 0.23 | 0.4 (0.3–0.7) | <0.0001 | −0.24 ± 0.26 | 0.8 (0.5–1.3) | 0.34 | −0.17 ± 0.11 | 0.8 (0.7–1.0) | 0.13 |
| No hypercholesterolemia | −0.11 ± 0.25 | 0.9 (0.5–1.5) | 0.66 | 0.22 ± 0.29 | 1.2 (0.7–2.2) | 0.44 | 0.12 ± 0.12 | 1.1 (0.9–1.4) | 0.30 |
| Low body mass index | 0.01 ± 0.10 | 1.0 (0.8–1.2) | 0.91 | 0.18 ± 0.11 | 1.2 (0.9–1.5) | 0.10 | 0.05 ± 0.05 | 1.0 (0.9–1.1) | 0.28 |
| No hypertension | 0.68 ± 0.24 | 2.0 (1.2–3.2) | 0.004 | 0.45 ± 0.26 | 1.6 (0.9–2.6) | 0.08 | 0.15 ± 0.10 | 1.2 (0.9–1.4) | 0.14 |
| No diabetes mellitus | 0.94 ± 0.33 | 2.5 (1.3–4.9) | 0.004 | 0.70 ± 0.34 | 2.0 (0.9–4.0) | 0.04 | 0.40 ± 0.11 | 1.5 (1.2–1.9) | 0.0006 |
| No physical activity | −0.47 ± 0.43 | 0.6 (0.3–1.4) | 0.27 | −0.05 ± 0.46 | 0.9 (0.4–2.3) | 0.91 | −0.07 ± 0.22 | 0.9 (0.6–1.4) | 0.73 |
| No alcohol use | −0.13 ± 0.26 | 0.9 (0.5–1.5) | 0.61 | −0.32 ± 0.29 | 0.7 (0.4–1.3) | 0.28 | 0.04 ± 0.12 | 1.0 (0.8–1.3) | 0.72 |
| No coffee use | −0.74 ± 0.42 | 0.5 (0.2–1.1) | 0.07 | −0.35 ± 0.44 | 0.7 (0.3–1.7) | 0.42 | −0.15 ± 0.15 | 0.9 (0.6–1.2) | 0.32 |
| No family coronary heart disease | 0.02 ± 0.27 | 1.0 (0.6–1.7) | 0.95 | 0.30 ± 0.28 | 1.4 (0.8–2.4) | 0.28 | 0.11 ± 0.12 | 1.1 (0.9–1.4) | 0.33 |
| No angina (before enrollment) | 1.52 ± 0.44 | 4.5 (1.9–10.7) | 0.001 | 1.21 ± 0.45 | 3.3 (1.4–8.1) | 0.007 | 0.40 ± 0.12 | 1.5 (1.2–1.9) | 0.001 |
| No myocardial infarction (before enrollment) | 1.43 ± 0.41 | 4.2 (1.9–9.3) | <0.0001 | 1.35 ± 0.42 | 3.8 (1.7–8.8) | 0.002 | 0.41 ± 0.12 | 1.5 (1.2–1.9) | 0.0007 |
| Short prehospital time delay | 0.27 ± 0.10 | 1.3 (1.1–1.6) | 0.01 | 0.11 ± 0.11 | 1.1 (0.9–1.4) | 0.32 | 0.05 ± 0.04 | 1.0 (0.9–1.1) | 0.25 |
| Low systolic blood pressure | 0.18 ± 0.10 | 1.2 (1.0–1.5) | 0.07 | 0.02 ± 0.11 | 1.0 (0.8–1.3) | 0.87 | −0.01 ± 0.05 | 0.9 (0.8–1.1) | 0.76 |
| Low diastolic blood pressure | 0.02 ± 0.10 | 1.0 (0.8–1.2) | 0.85 | −0.06 ± 0.11 | 0.9 (0.8–1.2) | 0.58 | −0.04 ± 0.04 | 0.9 (0.8–1.0) | 0.34 |
| Low heart rate | 0.24 ± 0.10 | 1.3 (1.0–1.6) | 0.01 | 0.15 ± 0.11 | 1.2 (0.9–1.4) | 0.16 | 0.10 ± 0.05 | 1.2 (1.1–1.3) | 0.03 |
| Nonanterior myocardial infarction | −0.24 ± 0.24 | 0.8 (0.5–1.2) | 0.31 | −0.21 ± 0.25 | 0.8 (0.5–1.3) | 0.40 | −0.03 ± 0.11 | 0.9 (0.8–1.2) | 0.78 |
| Non–Q-wave myocardial infarction | −0.74 ± 0.30 | 0.5 (0.3–0.9) | 0.01 | −0.55 ± 0.32 | 0.6 (0.3–1.1) | 0.08 | −0.16 ± 0.11 | 0.8 (0.7–1.1) | 0.15 |
| Low creatine kinase peak | −0.18 ± 0.10 | 0.8 (0.7–1.0) | 0.07 | −0.13 ± 0.11 | 0.9 (0.7–1.1) | 0.22 | −0.01 ± 0.05 | 0.9 (0.8–1.1) | 0.78 |
| Low creatine kinase-MB peak | −0.18 ± 0.10 | 0.8 (0.7–1.0) | 0.08 | −0.13 ± 0.11 | 0.9 (0.7–1.1) | 0.23 | −0.01 ± 0.04 | 0.9 (0.8–1.1) | 0.96 |
| Low Killip class | 0.99 ± 0.24 | 2.7 (1.7–4.3) | <0.0001 | 0.60 ± 0.25 | 1.8 (1.1–3.0) | 0.01 | 0.41 ± 0.08 | 1.5 (1.3–1.8) | <0.0001 |
| No tachyarrhythmias | −0.02 ± 0.27 | 1.0 (0.6–1.6) | 0.92 | −0.20 ± 0.28 | 0.8 (0.5–1.4) | 0.46 | −0.05 ± 0.12 | 0.9 (0.7–1.2) | 0.64 |
| No bradyarrhythmias | 0.18 ± 0.43 | 1.2 (0.5–2.8) | 0.68 | 0.16 ± 0.45 | 1.2 (0.5–2.8) | 0.72 | 0.31 ± 0.18 | 1.4 (0.9–1.9) | 0.09 |
| No atrial fibrillation/flutter | 1.70 ± 0.60 | 5.4 (1.7–17.8) | 0.005 | 1.30 ± 0.62 | 3.6 (1.1–12.3) | 0.03 | 0.34 ± 0.14 | 1.4 (1.1–1.8) | 0.01 |
| Low left ventricular ejection fraction | −0.46 ± 0.12 | 0.6 (0.5–0.8) | <0.0001 | −0.38 ± 0.13 | 0.7 (0.5–0.9) | 0.002 | −0.21 ± 0.05 | 0.8 (0.7–0.9) | <0.0001 |
| Low hemoglobin | −0.18 ± 0.10 | 0.8 (0.7–1.0) | 0.08 | −0.05 ± 0.12 | 0.9 (0.8–1.2) | 0.68 | −0.04 ± 0.05 | 1.0 (0.9–1.0) | 0.35 |
| Low blood glucose | 0.25 ± 0.10 | 1.3 (1.1–1.6) | 0.01 | 0.19 ± 0.11 | 1.2 (1.0–1.5) | 0.09 | 0.12 ± 0.04 | 1.1 (1.0–1.2) | 0.005 |
| Low total cholesterol | −0.06 ± 0.10 | 0.9 (0.8–1.1) | 0.53 | 0.05 ± 0.11 | 1.1 (0.8–1.3) | 0.62 | 0.02 ± 0.05 | 1.0 (0.9–1.1) | 0.70 |
| Low high-density lipoprotein cholesterol | −0.02 ± 0.10 | 1.0 (0.8–1.2) | 0.87 | −0.07 ± 0.11 | 0.9 (0.7–1.1) | 0.48 | −0.06 ± 0.04 | 0.9 (0.9–1.0) | 0.19 |
| Low triglycerides | −0.05 ± 0.10 | 0.9 (0.8–1.2) | 0.61 | 0.13 ± 0.11 | 1.1 (0.9–1.4) | 0.23 | 0.10 ± 0.05 | 1.2 (1.1–1.3) | 0.03 |
| Low potassium | 0.07 ± 0.10 | 1.1 (0.9–1.3) | 0.51 | −0.02 ± 0.11 | 0.9 (0.8–1.2) | 0.82 | 0.02 ± 0.04 | 1.0 (0.9–1.1) | 0.59 |
| Low uric acid | 0.21 ± 0.10 | 1.2 (1.1–1.5) | 0.03 | 0.15 ± 0.11 | 1.2 (0.9–1.4) | 0.18 | 0.15 ± 0.04 | 1.2 (1.1–1.3) | 0.001 |
| Low estimated glomerular filtration rate | −0.64 ± 0.11 | 0.5 (0.4–0.7) | <0.0001 | −0.40 ± 0.13 | 0.7 (0.5–0.9) | 0.002 | −0.19 ± 0.05 | 0.8 (0.7–0.9) | 0.0002 |
| Low albumin/creatinine excretion ratio | 0.54 ± 0.11 | 1.7 (1.4–2.1) | <0.0001 | 0.35 ± 0.12 | 1.4 (1.1–1.8) | 0.004 | 0.25 ± 0.05 | 1.3 (1.2–1.4) | <0.0001 |
| Variable modification during follow-up | |||||||||
| No current smoking | −0.71 ± 0.23 | 0.5 (0.3–0.8) | 0.002 | −0.18 ± 0.26 | 0.8 (0.5–1.4) | 0.48 | −0.17 ± 0.11 | 0.8 (0.7–1.0) | 0.12 |
| No hypercholesterolemia | −0.54 ± 0.23 | 0.6 (0.4–0.9) | 0.01 | −0.11 ± 0.25 | 0.9 (0.5–1.5) | 0.65 | 0.03 ± 0.11 | 1.0 (0.8–1.3) | 0.77 |
| No hypertension | 0.46 ± 0.23 | 1.6 (1.1–2.5) | 0.04 | 0.20 ± 0.25 | 1.0 (0.7–2.0) | 0.81 | 0.01 ± 0.10 | 1.0 (0.8–1.2) | 0.93 |
| No diabetes mellitus | 0.75 ± 0.29 | 2.1 (1.2–3.7) | 0.009 | 0.66 ± 0.30 | 1.9 (1.1–3.5) | 0.02 | 0.33 ± 0.11 | 1.4 (1.1–1.7) | 0.002 |
| No physical activity | −1.09 ± 0.24 | 0.3 (0.2–0.5) | <0.0001 | −0.46 ± 0.27 | 0.6 (0.4–1.1) | 0.08 | −0.21 ± 0.13 | 0.8 (0.6–1.0) | 0.10 |
At the multivariate level, both the logistic and the Cox regression models showed independent associations between EFS and age, no history of angina and/or myocardial infarction, high eGFR, and low albumin/creatinine ratio ( Table 3 , model 1). Only the Cox model showed significant associations between EFS and the absence of diabetes mellitus and low Killip class. All other variables, except for LVEF, showed a weak or no association with EFS, including the major CV risk factors ( Table 3 , model 2). Figure 3 shows the proportion of patients with EFS according to quartiles or classes of variables found significant in the predictive models. Independent significant interactions were found between age and the albumin/creatinine ratio, age and LVEF, Killip class and eGFR, Killip class and albumin/creatinine ratio, and diabetes mellitus and history of angina ( Table 3 , interaction section).
| Variable | β ± SE | OR (95% CI) | p Value | β ± SE | HR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Model 1 | All patients (n = 504) | |||||
| Age | 0.57 ± 0.14 | 1.8 (1.4–2.4) | <0.0001 | 0.19 ± 0.05 | 1.2 (1.1–1.3) | 0.0006 |
| Women | −0.37 ± 0.33 | 0.7 (0.4–1.3) | 0.25 | 0.09 ± 0.12 | 1.1 (0.8–1.4) | 0.47 |
| Angina pectoris | 0.98 ± 0.48 | 2.6 (1.1–6.8) | 0.03 | 0.35 ± 0.13 | 1.4 (1.1–1.8) | 0.009 |
| Estimated glomerular filtration rate | −0.35 ± 0.13 | 0.7 (0.5–0.9) | 0.009 | −0.16 ± 0.05 | 0.8 (0.7–0.9) | 0.001 |
| Albumin/creatinine ratio | 0.36 ± 0.13 | 1.4 (1.1–1.8) | 0.005 | 0.20 ± 0.05 | 1.2 (1.1–1.3) | <0.0001 |
| Previous myocardial infarction | 0.99 ± 0.45 | 2.7 (1.1–6.5) | 0.02 | 0.31 ± 0.12 | 1.4 (1.1–1.7) | 0.01 |
| Highest Killip class | 0.25 ± 0.09 | 1.3 (1.1–1.5) | 0.004 | |||
| Diabetes mellitus | 0.24 ± 0.12 | 1.3 (1.1–1.6) | 0.04 | |||
| Model 2 | Patients with LVEF (n = 401) | |||||
| Age | 0.53 ± 0.16 | 1.7 (1.2–2.3) | 0.001 | 0.21 ± 0.06 | 1.2 (1.1–1.4) | 0.001 |
| Women | −0.55 ± 0.35 | 0.6 (0.3–1.1) | 0.11 | −0.11 ± 0.13 | 0.9 (0.7–1.2) | 0.38 |
| Estimated glomerular filtration rate | −0.36 ± 0.15 | 0.7 (0.5–0.9) | 0.01 | −0.16 ± 0.06 | 0.8 (0.7–0.9) | 0.004 |
| Albumin/creatinine ratio | 0.37 ± 0.14 | 1.4 (1.1–1.9) | 0.01 | 0.21 ± 0.06 | 1.2 (1.1–1.4) | 0.0002 |
| Angina pectoris | 1.18 ± 0.51 | 3.3 (1.2–8.8) | 0.02 | |||
| Left ventricular ejection fraction | −0.29 ± 0.13 | 0.7 (0.6–0.9) | 0.03 | −0.14 ± 0.05 | 0.8 (0.7–0.9) | 0.006 |
| Interactions | ||||||
| Age*albumin/creatinine ratio | 0.33 ± 0.12 | 1.4 (1.1–1.8) | 0.009 | 0.11 ± 0.04 | 1.2 (1.1–1.3) | 0.008 |
| Age*left ventricular ejection fraction | −0.29 ± 0.14 | 0.7 (0.6–0.9) | 0.03 | |||
| Killip class*estimated glomerular filtration rate | −0.69 ± 0.28 | 0.5 (0.3–0.9) | 0.01 | |||
| Killip class*albumin/creatinine ratio | 0.17 ± 0.07 | 1.2 (1.1–1.4) | 0.02 | |||
| Diabetes mellitus*angina pectoris | 0.54 ± 0.26 | 1.7 (1.1–2.9) | 0.03 | |||
| Model 3 | Patients alive at hospital discharge (n = 464) | |||||
| Age | 0.54 ± 0.14 | 1.7 (1.3–2.3) | <0.0001 | 0.18 ± 0.06 | 1.2 (1.1–1.3) | 0.001 |
| Women | −0.01 ± 0.34 | 1.0 (0.5–1.9) | 0.95 | 0.11 ± 0.13 | 1.1 (0.9–1.4) | 0.38 |
| Angina pectoris | 1.00 ± 0.47 | 2.7 (1.1–6.9) | 0.03 | 0.41 ± 0.14 | 1.5 (1.1–2.0) | 0.004 |
| Previous myocardial infarction | 0.99 ± 0.45 | 2.7 (1.1–6.9) | 0.02 | 0.31 ± 0.13 | 1.4 (1.0–1.8) | 0.02 |
| Estimated glomerular filtration rate | −0.33 ± 0.13 | 0.7 (0.6–0.9) | 0.01 | −0.14 ± 0.05 | 0.8 (0.7–0.9) | 0.005 |
| Albumin/creatinine ratio | 0.34 ± 0.13 | 1.4 (1.1–1.8) | 0.009 | 0.16 ± 0.05 | 1.2 (1.1–1.3) | 0.001 |
| Diabetes mellitus | 0.27 ± 0.12 | 1.3 (1.1–1.7) | 0.03 | |||
| Model 4 | Only CV events (n = 504) | |||||
| Age | 0.29 ± 0.11 | 1.3 (1.1–1.7) | 0.01 | 0.14 ± 0.06 | 1.2 (1.1–1.3) | 0.01 |
| Women | 0.02 ± 0.28 | 1.0 (0.6–1.8) | 0.93 | 0.09 ± 0.13 | 1.1 (0.8–1.4) | 0.48 |
| Angina pectoris | 0.71 ± 0.32 | 2.0 (1.1–3.8) | 0.02 | 0.36 ± 0.14 | 1.4 (1.1–1.9) | 0.01 |
| Previous myocardial infarction | 0.66 ± 0.31 | 1.9 (1.1–3.5) | 0.03 | 0.28 ± 0.12 | 1.3 (1.1–1.7) | 0.02 |
| Albumin/creatinine ratio | 0.25 ± 0.11 | 1.3 (1.1–1.6) | 0.02 | 0.19 ± 0.05 | 1.2 (1.1–1.3) | 0.0002 |
| Estimated glomerular filtration rate | −0.23 ± 0.10 | 0.8 (0.6–0.9) | 0.02 | −0.15 ± 0.05 | 0.8 (0.7–0.9) | 0.005 |
| Diabetes mellitus | 0.75 ± 0.30 | 2.1 (1.2–3.8) | 0.01 | 0.29 ± 0.12 | 1.3 (1.1–1.7) | 0.01 |
| Highest Killip class | 0.65 ± 0.23 | 1.9 (1.2–3.0) | 0.004 | 0.30 ± 0.09 | 1.3 (1.1–1.6) | 0.0008 |
| Hypertension | 0.46 ± 0.23 | 1.6 (1.1–2.5) | 0.04 | |||
| Left ventricular ejection fraction | −0.11 ± 0.05 | 0.9 (0.8–1.1) | 0.04 | |||
| Model 5 | Inclusion of in-hospital medications (n = 504) | |||||
| Age | 0.50 ± 0.16 | 1.6 (1.2–2.3) | 0.002 | 0.18 ± 0.06 | 1.2 (1.1–1.3) | 0.002 |
| Women | −0.15 ± 0.36 | 0.9 (0.4–1.7) | 0.67 | 0.06 ± 0.13 | 1.1 (0.8–1.4) | 0.65 |
| Angina pectoris | 0.96 ± 0.47 | 2.6 (1.1–6.5) | 0.04 | 0.47 ± 0.13 | 1.6 (1.2–2.0) | 0.0003 |
| Diabetes mellitus | 0.33 ± 0.16 | 1.4 (1.1–1.9) | 0.03 | |||
| Previous myocardial infarction | 0.92 ± 0.47 | 2.5 (1.1–6.3) | 0.04 | |||
| Estimated glomerular filtration rate | −0.42 ± 0.14 | 0.7 (0.5–0.9) | 0.003 | −0.18 ± 0.05 | 0.8 (0.7–0.9) | 0.0005 |
| Albumin/creatinine ratio | 0.37 ± 0.14 | 1.4 (1.1–1.9) | 0.007 | 0.19 ± 0.05 | 1.2 (1.1–1.3) | 0.0001 |
| Left ventricular ejection fraction | −0.33 ± 0.15 | 0.7 (0.5–0.9) | 0.02 | −0.12 ± 0.06 | 0.8 (0.7–0.9) | 0.03 |
| Model 6 | Inclusion of medications during follow-up (n = 464) | |||||
| Age | 0.42 ± 0.16 | 1.5 (1.1–2.1) | 0.008 | 0.13 ± 0.06 | 1.2 (1.1–1.3) | 0.04 |
| Women | −0.28 ± 0.36 | 0.8 (0.4–1.5) | 0.43 | 0.08 ± 0.13 | 1.1 (0.8–1.4) | 0.55 |
| Angina pectoris | 1.07 ± 0.45 | 2.9 (1.2–7.1) | 0.01 | 0.41 ± 0.14 | 1.5 (1.1–2.0) | 0.004 |
| Previous myocardial infarction | 0.26 ± 0.14 | 1.3 (1.0–1.7) | 0.06 | |||
| Estimated glomerular filtration rate | −0.34 ± 0.13 | 0.7 (0.5–0.9) | 0.01 | −0.15 ± 0.05 | 0.8 (0.7–0.9) | 0.004 |
| Albumin/creatinine ratio | 0.28 ± 0.13 | 1.3 (1.1–1.7) | 0.03 | 0.14 ± 0.05 | 1.2 (1.1–1.3) | 0.006 |
| Model 7 | Inclusion of CV risk factor modification during follow-up (n = 504) | |||||
| Age | 0.52 ± 0.15 | 1.7 (1.2–2.3) | 0.001 | 0.18 ± 0.06 | 1.2 (1.1–1.4) | 0.004 |
| Women | −0.03 ± 0.35 | 1.0 (0.5–1.9) | 0.92 | 0.12 ± 0.14 | 1.1 (0.9–1.5) | 0.39 |
| Angina pectoris | 1.04 ± 0.44 | 2.8 (1.2–6.7) | 0.01 | 0.40 ± 0.14 | 1.5 (1.1–1.9) | 0.005 |
| Previous myocardial infarction | 1.03 ± 0.41 | 2.8 (1.2–6.3) | 0.01 | 0.34 ± 0.14 | 1.4 (1.1–1.8) | 0.01 |
| Estimated glomerular filtration rate | −0.34 ± 0.13 | 0.7 (0.5–0.9) | 0.01 | −0.15 ± 0.05 | 0.8 (0.7–0.9) | 0.003 |
| Albumin/creatinine ratio | 0.30 ± 0.13 | 1.3 (1.1–1.7) | 0.02 | 0.17 ± 0.05 | 1.2 (1.1–1.3) | 0.0009 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


