We sought to determine whether preoperative baseline echocardiographic analysis and the type of surgical procedure are predictive of the magnitude and timing of postoperative left ventricular (LV) remodeling in patients undergoing valve surgery for pure severe mitral regurgitation (MR) secondary to leaflet prolapse. Seventy-two consecutive patients without coronary artery disease undergoing valve repair (MVr; n = 42) or replacement (MVR; n = 30) underwent preoperative, early (1 to 2 days) and late postoperative (4.5 ± 2.5 and 18 ± 8.0 months) echocardiography. Patients were categorized according to their baseline LV ejection fraction (EF) (Group 1: EF ≥60%, Group 2: EF = 50% to 59%, Group 3: EF <50%). Preservation of the subvalvular apparatus was achieved in most patients undergoing MV replacement (87%). Over a median follow-up period of 450 days, LVEF changed as follows: Group 1: 63% ± 2% to 60% ± 3% (p <0.0001); Group 2: 55% ± 3% to 52% ± 6% (p <0.0001); Group 3: 43% ± 4% to 42% ± 5% (p <0.01). Two-thirds of the observed changes in LV diameters and volumes occurred in the first 6 months. Preoperative LVEF was the best predictor of postoperative LVEF ≥60% (odds ratio 1.50, 95% confidence interval, 1.25 to 1.97; p <0.0001). No significant difference was found in LV remodeling parameters between patients undergoing MVr and MVR. In conclusion, patients with pure severe MR due to valve prolapse LVEF remained normal after surgery only in patients with baseline LVEF ≥60%. MVR with subvalvular preservation was associated with similar postoperative remodeling as MVr.
Organic mitral disease and especially mitral valve prolapse (MVP) is the main cause of mitral regurgitation (MR) in the Western world and the main indication for mitral valve surgery in the United States. Significant MR leads to progressive deterioration in left ventricular (LV) function as well as serious morbidity and mortality if patient are treated medically, but the extent of LV function recovery after correction of MR remains unclear. Additionally, the timing and magnitude of LV remodeling after surgery in patients with pure severe MR is still poorly described. Previous studies have shown that preoperative left ventricular ejection fraction (LVEF) and LV end-systolic diameter can best predict LV remodeling and outcomes if either medical treatment or surgical correction was chosen as therapy of choice. However, these studies often included heterogeneous populations, especially patients with concomitant significant coronary artery disease. Furthermore, these data were acquired during an earlier time period (1980s and 1990s) when surgical techniques and pharmacologic medical treatment were different and may not reflect the contemporary results of current practices. We sought to determine whether preoperative echocardiographic analysis and the type of surgical procedure are predictive of the timing and magnitude of postoperative left ventricular remodeling in patients undergoing valve surgery for pure severe MR secondary to leaflet prolapse.
Methods
We retrospectively analyzed 72 consecutive patients who underwent mitral valve repair (MVr) or replacement (MVR) at our tertiary care center (Institut Universitaire de Cardiologie et de Pneumologie de Québec, Quebec, Canada) between March 2005 and July 2010 for symptomatic pure severe mitral valve regurgitation secondary to MVP. We excluded patients with significant coronary artery disease or concomitant aortic valve disease. Patients were categorized in 3 groups according to their respective baseline LVEF (Group 1: EF ≥60%, Group 2: EF = 50% to 59%, Group 3: EF <50%). This study was approved by our institution review board and ethics committee.
Two-dimensional and Doppler transthoracic echocardiography examinations were performed using Philips Medical Systems (Amsterdam, Netherlands) platforms. Two hundred eighty-eight 2-dimensional echocardiograms were retrospectively analyzed by 2 experienced investigators (MS and KO) via Xcelera Echo Lab Management (Amsterdam, Netherlands). There was an excellent correlation (r ≥0.95) between intraobserver and interobserver measurement for all parameters as estimated by Pearson coefficient of correlation. Intraobserver and interobserver relative difference were <5% for all parameters (range for intraobserver 0.8% to 5%; interobserver 1% to 4%).
All patients had preoperative (Echo 1), early postoperative (1 to 2 days; Echo 2), late postoperative (mean of 4.5 ± 2.5 months [Echo 3] and 18 ± 8.0 months [Echo 4]) echocardiographic studies. LVEF and volumes were averaged values from the apical 4-chamber and 2-chamber views and calculated using the modified biplane Simpson method. LV dimensions were measured using M-mode technique via the parasternal-long axis view. Finally, normal LVEF was defined as a LVEF ≥60%.
Both MVr and MVR were done either through a median sternotomy or a minimally invasive right thoracotomy, with the use of cardiopulmonary bypass, mild hypothermia, and cold-blood intermittent cardioplegia. MVr procedures were performed with different technical approaches to suit anatomic particularities but involved a downsizing rigid ring annuloplasty in all cases. Although most patients underwent quadrangular P2 resection, additional techniques including partial or complete resection of other leaflet segments, as well as chordal transposition or transfer, neochordae implantation, and leaflet sliding plasty, were undertaken as required. MVR were performed with preservation of the subvalvular apparatus whenever possible. The posterior leaflet was preserved by incorporation of the entire leaflet tissue within the pledgetted mattress braided sutures used for prosthesis anchoring to the annulus. Anterior leaflet preservation was performed by dividing its mid-anterior portion all the way to the edge, creating 2 hemi-leaflets from which all chordae were left intact. These structures were then plicated on the annulus at the level of the corresponding commissures using the pledgetted braided valve-anchoring sutures, or additional polypropylene sutures according to surgeons’ preferences.
Categorical variables were expressed as numbers and percentages and continuous variables as mean ± SD. Categorical parameters were compared using Fischer’s exact test or chi-square test for categorical variables. For continuous variables, Student’s t test, Wilcoxon test, or 1-way analysis of variance was used as appropriate. Predictors of postoperative LV function were selected with stepwise, backward and forward procedures with logistic regression analyses. Stepwise selection was used to identify potential predictors, which were entered into the model at p <0.1 and retained at p <0.1. Finally, a p value of <0.05 was considered significant. All statistical tests were performed using JMP 9.0.0 (SAS Institute, Cary, North Carolina).
Results
Seventy-two patients, with a mean age of 60 ± 13 years, underwent MVr (n = 42, 58%) or MVR (n = 30, 42%; Table 1 ). Fifty-three (74%) were men. Median follow-up was 450 days (range: 252 to 1,329 days). Characterization of MVP was based on preoperative echocardiography and corroborated with the intraoperative findings. Posterior leaflet prolapse was observed in 41 patients (57%); 23 (32%) and 8 patients (11%) had bileaflet prolapse and anterior leaflet prolapse, respectively. Fifteen patients (21%) had a complete flail (eversion of the leaflet tip into the left atrium) mitral valve. Intergroup comparison revealed no statistically significant difference other than baseline echocardiographic parameters ( Table 1 ). Table 2 depicts the surgical characteristics according to type of mitral surgery. In patients with MVR, 83% had a mechanical prosthesis, and 87% had partial or complete preservation of their mitral apparatus. Moreover, half of our entire cohort had a right mini-thoracotomy.
| Variable | All (n = 72) | LVEF | p Value | ||
|---|---|---|---|---|---|
| ≥60% (n = 29) | 50%–59% (n = 26) | <50% (n = 17) | |||
| Age (mean) | 60.2 ± 13.0 | 59.6 ± 2.5 | 61.5 ± 2.6 | 59.1 ± 3.2 | 0.82 |
| Male patients | 53 (74%) | 20 (69%) | 18 (69%) | 15 (88%) | 0.29 |
| Body mass index (kg/m 2 ) | 24.3 ± 3.4 | 24.2 ± 3.1 | 23.8 ± 2.8 | 25.3 ± 4.5 | 0.35 |
| MVP | |||||
| Posterior | 41 (57%) | 15 (52%) | 14 (54%) | 12 (71%) | 0.42 |
| Anterior | 8 (11%) | 2 (7%) | 4 (15%) | 2 (12%) | 0.60 |
| Bileaflet | 23 (32%) | 12 (41%) | 8 (31%) | 3 (18%) | 0.25 |
| Flail ∗ | 15 (21%) | 4 (14%) | 7 (27%) | 4 (24%) | 0.47 |
| MVr | 42 (58%) | 17 (58%) | 15 (58%) | 10 (58%) | 0.94 |
| Preoperative LVEF (%) | 55 ± 8 | 63 ± 3 | 55 ± 3 | 43 ± 4 | <0.0001 |
| Preoperative LVEDD (mm) | 58 ± 7 | 56 ± 4 | 57 ± 5 | 63 ± 9 | <0.0001 |
| Preoperative LVESD (mm) | 41 ± 8 | 37 ± 5 | 39 ± 6 | 50 ± 8 | <0.0001 |
| Preoperative LVEDV (ml) | 160 ± 47 | 147 ± 33 | 154 ± 48 | 192 ± 53 | 0.0042 |
| Preoperative LVESV (ml) | 72 ± 30 | 54 ± 13 | 69 ± 22 | 108 ± 33 | <0.0001 |
∗ Defined as complete eversion of the leaflet tip into the left atrium.
| Variable | MVR (n = 30) | MVr (n = 42) |
|---|---|---|
| Prosthesis type | ||
| Mechanical | 25 (83%) | — |
| Bioprosthesis | 5 (17%) | — |
| Rigid ring | — | 42 (100%) |
| Prosthesis size (mm) | 31.7 ± 2.4 | 31.2 ± 3.1 |
| Surgical approach | ||
| Median sternotomy | 18 (60%) | 17 (40%) |
| Right minithoracotomy | 12 (40%) | 25 (60%) |
| Subvalvular apparatus preservation | ||
| Yes | 26 (87%) | — |
| Complete | 16 (54%) | — |
| Posterior only | 9 (30%) | — |
| Anterior only | 1 (3%) | — |
| No | 4 (13%) | — |
| Mitral valve repair techniques | ||
| P2 resection | — | 18 (43%) |
| P2 resection with sliding plasty | — | 9 (21%) |
| P2 resection with chordal transfer | — | 4 (10%) |
| P2 resection with sliding plasty and chordal transfer | — | 1 (2%) |
| Annuloplasty only | — | 3 (7%) |
| Neochordae only | — | 2 (5%) |
| Alfieri ± leaflet resection | — | 2 (5%) |
| Chordal transfer only | — | 2 (5%) |
| Anterior and posterior resection | — | 1 (2%) |
In all patients, LVEF reached its lowest value in the early postoperative period with a gradually positive remodeling ensuing thereafter but never equaling or exceeding the baseline value (Echo 1: 56% ± 8% vs Echo 4: 53% ± 9% [p <0.0001]). LV diameters and volumes decreased continuously during the follow-up period (LV end-diastolic diameter: 58.0 ± 8.0 mm to 47.0 ± 5.0 mm [p <0.0001]; LV end-systolic diameter: 41.0 ± 8.0 mm to 33.0 ± 6.0 mm [p <0.0001]; LV end-diastolic volume: 160 ± 47 ml to 104 ± 26 ml [p <0.0001]; LV end-systolic volume: 72 ± 30 ml to 50 ± 19 ml [p <0.0001]).
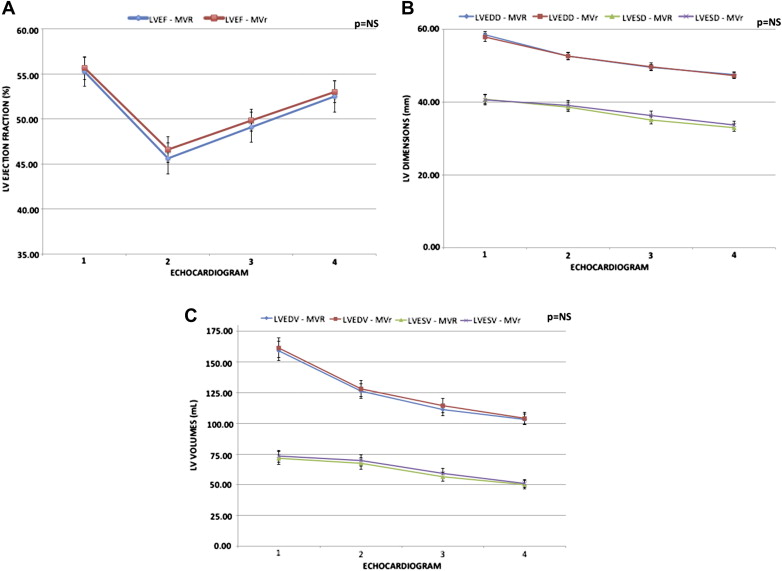
At follow-up, subgroup analysis based on preoperative LVEF showed similar variations in LVEF, LV diameters, and LV volumes (end-diastolic and end-systolic). Patients with preoperative LVEF ≥60% showed an early postoperative decrease in LVEF but remodeled positively thereafter to a normal LVEF (63% ± 2% to 60% ± 3% [p <0.0001]). In comparison, the 2 other groups were unable to normalize their LVEF at last follow-up despite similar positive remodeling (Group 2: 55% ± 3% to 52% ± 6% [p <0.0001]; Group 3: 44% ± 4% to 43% ± 5% [p <0.0001]); ( Figure 1 ). Furthermore, a continuous and progressive decrease in the LV volumetric and dimensional parameters was observed over the follow-up period. Two-thirds of the entire remodeling occurred in the first 6 months, with smaller but nonetheless additional remodeling thereafter ( Figure 1 ).
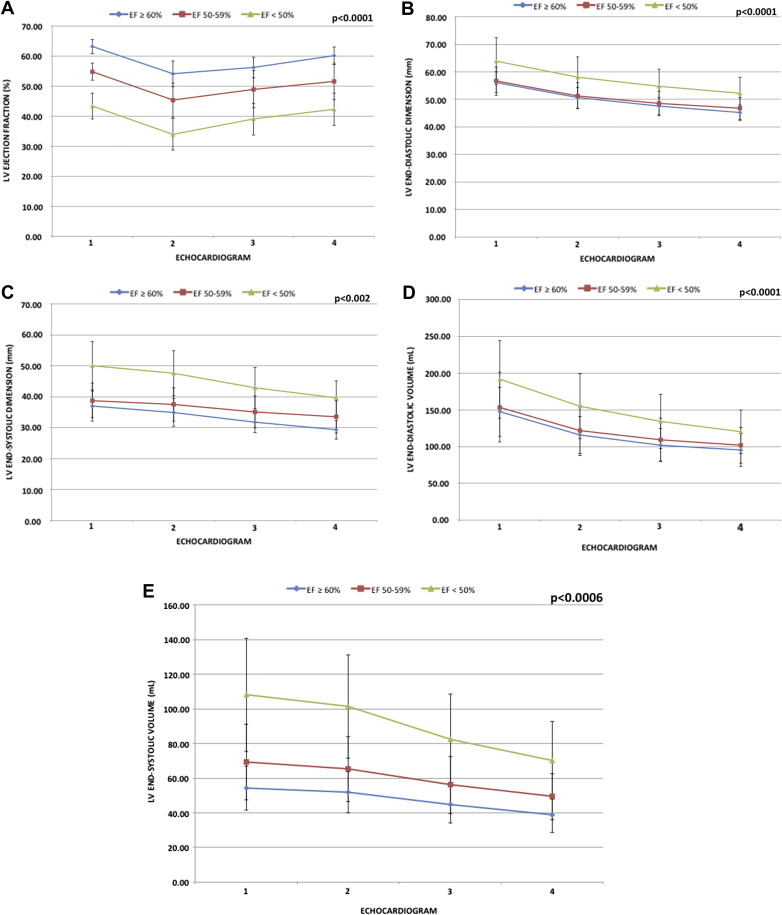
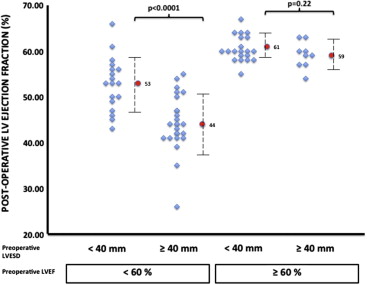
Regardless of preoperative LV end-systolic diameter (<40 vs ≥40 mm), patients with a preoperative LVEF ≥60% had similar LVEF at last follow-up. In contrast, patients with preoperative LVEF <60% had LVEF at last follow-up significantly lower in the LV end-systolic diameter ≥40 mm subgroup compared with patients within the LV end-systolic diameter <40 mm subgroup ( Figure 2 ).
The preoperative presence of a LVEF ≥60% had the best performance for the prediction of a postoperative LVEF ≥60% at last follow-up: sensitivity 90%, specificity 77%, positive and negative predictive values of 59% and 95%, respectively. Preoperative systolic diameter <40 mm had an acceptable sensitivity and negative predictive value of 79% and 88% but a low specificity and positive predictive value of 57% and 40% respectively. Throughout follow-up, no significant difference was found in echocardiography remodeling parameters between patients who underwent MVR compared with MVr ( Figure 3 ).