Early in the course of ST-segment elevation myocardial infarction (STEMI), therapies that may harm patients who develop left ventricular (LV) dysfunction, such as β-blockers, are often administered. The investigators analyzed the ACTIVATE-SF database, a registry of consecutive STEMI activations presenting to 2 medical centers at the University of California, San Francisco. LV dysfunction was defined as an ejection fraction ≤40% on echocardiography. Of 211 patients included in the analysis, 66 (31%) had LV ejection fractions ≤40%. Patients with LV dysfunction were older (63 ± 15 vs 56 ± 13 years, p = 0.002). In multivariate regression models, decreased renal function (reference group, creatinine <1.0 mg/dl; adjusted odds ratio [AOR] creatinine >1.5 mg/dl 6.35, 95% confidence interval [CI] 1.66 to 24.31, p = 0.007), a history of coronary artery disease (AOR 3.12, 95% CI 1.26 to 7.71, p = 0.014), ST-segment elevation >2 mm on 12-lead electrocardiography (AOR 2.78, 95% CI 1.31 to 5.87, p = 0.008), and need for mechanical ventilation (AOR 3.98, 95% CI 1.41 to 11.19, p = 0.009) increased the odds of LV dysfunction. Inferior ST-segment elevations were associated with 88% decreased odds of LV dysfunction (AOR 0.12, 95% CI 0.06 to 0.35, p <0.001). A prediction score using these characteristics stratified patients into low-, intermediate-, and high-risk groups for LV dysfunction; positive likelihood ratios for LV dysfunction in these groups were 0.07, 1.14, and 4.93, respectively. In conclusion, 5 key predictors of in-hospital LV dysfunction after STEMI were identified; a risk score based on these predictors helps to quickly identify patients presenting with STEMI who are at the highest risk for developing significant LV dysfunction and could guide optimal therapeutic choices.
Although the incidence of ST-segment elevation myocardial infarction (STEMI) has decreased over the past decade, it remains a common and morbid diagnosis. Early identification, preferably in the emergency department (ED), of patients at highest risk for developing left ventricular (LV) dysfunction could serve to inform the use of certain therapies, such as β blockers and intravenous fluid resuscitation, which are potentially harmful in patients with STEMI and LV dysfunction. Identifying these high-risk patients in the ED may also facilitate more appropriate use of postinfarction therapies, including eplerenone and implantable cardioverter-defibrillators. We sought to describe important predictors of decreased LV systolic performance in patients presenting with STEMI and to develop a prediction model to help identify the patients at highest risk for developing LV dysfunction.
Methods
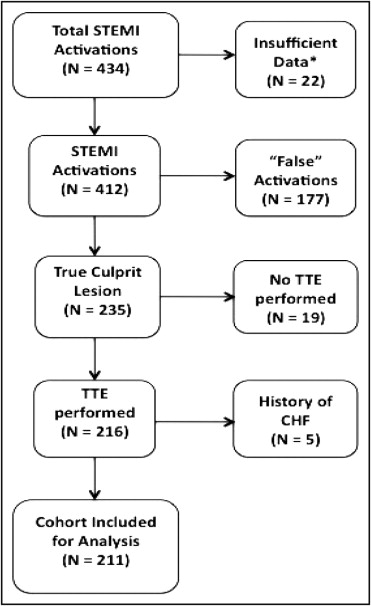
The ACTIVATE-SF registry is composed of a tertiary care university hospital (the University of California, San Francisco) and an urban county hospital and trauma center (San Francisco General Hospital). All ED physician–initiated STEMI activations were recorded in the ACTIVATE-SF registry irrespective of outcome from October 2008 to April 2011. Subject collection was based on hospital quality records compiled for the Joint Commission and was cross-referenced against the STEMI pager call logs and through weekly communication with the interventional cardiologists at each hospital. Among the 434 total STEMI activations during our study period, all patients who were brought to the catheterization laboratory were tracked, irrespective of angiographic results. Among the 84 patients who did not receive diagnostic angiography, only 22 (5% of the total population) lacked sufficient identifying data to be included in the registry.
For the purposes of this analysis, we selected the portion of the cohort that had true STEMI. True STEMI was defined by the presence of a culprit artery (i.e., a total or subtotal thrombotic occlusion of a coronary artery that accounted for the diagnostic electrocardiographic abnormalities; Figure 1 ). Given that the outcome was predicated on postevent LV function, we excluded patients who carried previous diagnoses of congestive heart failure. Patients who did not undergo echocardiography (19 of 434) during the index hospitalization were excluded. Patients who had acute coronary syndromes (including STEMI) before the index STEMI were included in the analysis.
All clinical data on patient presentation were collected from the ED physician and nursing notes. The inciting STEMI electrocardiogram (i.e., the tracing that led to the decision to activate the STEMI team) was deidentified and reread for key variables by 2 cardiologists blinded to clinical outcomes. Laboratory values and angiographic and echocardiographic data were collected from the electronic medical records. Study data were collected and managed using REDCap electronic data capture tools hosted at University of California, San Francisco.
ST-segment elevation was defined as J-point elevation in ≥2 contiguous leads of ≥2 mm in leads V 1 , V 2 , or V 3 and ≥1 mm in other leads. ST-segment depression ≥1 mm in leads V 1 to V 3 , consistent with a posterior STEMI, was also considered ST-segment elevation. A single territory of ST-segment elevation was assigned for each patient on the basis of the territory that displayed the greatest magnitude of ST-segment elevation. A culprit coronary artery was defined as noted previously. Mechanical ventilation was defined as the need for tracheal intubation and mechanical ventilation during the patient’s stay in the ED. History of coronary artery disease was ascertained by reviewing the charting of the ED physicians; any mention of previous acute coronary syndromes or simply listing coronary artery disease in the medical history qualified as a history of coronary artery disease.
Two-dimensional echocardiography was used for assessment of the LV ejection fraction. All studies were performed during the index hospitalization, <72 hours after the onset of the myocardial infarction. Volumes were estimated by averaging those derived from the 2-chamber and 4-chamber views according to Simpson’s method, and the ejection fraction was calculated in standard fashion.
The main outcome measure, depressed LV function, was defined as an LV ejection fraction ≤40% as determined by echocardiography. The ejection fraction cut point of 40% was chosen before the analysis; 40% represents a clinically meaningful delineation between mild LV dysfunction (or normal LV function) and moderate to severe LV dysfunction.
Summary statistics for the overall sample were constructed by using frequencies and proportions for categorical data and means, SDs, medians, and interquartile ranges for continuous variables. These summary statistics were stratified by whether patients had depressed LV function.
Univariate logistic regression was used to determine the unadjusted relations (odds ratios) between predictor variables and depressed LV function. Next, a multivariate logistic regression model was constructed using depressed LV function as the outcome. Two methods were used to determine which covariates to include in the model. First, a group of likely confounders (e.g., age) was specified a priori; these were automatically included in the model for face validity. Second, another list of possible confounders was generated using a directed acyclic graph, which was constructed from general clinical knowledge and data from previous studies. Confounders from this second group were kept in the model if they were found to be statistically significantly (p <0.10) associated with the outcome; a manual stepwise elimination algorithm was used. Interaction terms chosen for clinical or biologic plausibility were added to the base model and tested for statistical significance (p <0.10). The final model provided adjusted odds ratios describing the relations between clinical variables (patient characteristics, laboratory values, electrocardiographic findings) and depressed LV function.
Continuous variables (i.e., age and baseline creatinine) were assessed with regard to assumptions of linearity. Creatinine was broken into 3 categories to improve model fit. Model fit was formally assessed using the Hosmer-Lemeshow goodness-of-fit test.
A prediction score was developed to provide a schema for the assessment of a patient’s likelihood of having depressed LV function using clinical variables readily accessible in the ED setting. Key predictors from the multivariate logistic regression model were included in an empiric initial prediction score. Coefficients were modified and cutoffs were established with the design of maximizing the sensitivity of the prediction score. Fit was assessed using a receiver-operating characteristic curve with associated measurements of area under the curve. All analyses were performed using Stata version 10 (StataCorp LP, College Station, Texas).
Results
A total of 211 patients were included in the analysis ( Table 1 ). Of these, 66 patients (31%) had depressed LV function. Patients with depressed LV function were older (62.7 ± 15.1 vs 56.5 ± 12.8 years, p = 0.002), had higher creatinine (1.22 ± 0.69 vs 1.00 ± 0.34 mg/dl, p = 0.002), and were more likely to have anterior ST-segment elevation (59% vs 27%, p <0.001) than those with preserved LV function. Gender, race, and chronic co-morbidities were not significantly different between the 2 groups. Of note, door-to-balloon time and the success rate of percutaneous coronary intervention did not differ between the 2 groups.
| Characteristic | LV Ejection Fraction | ||
|---|---|---|---|
| ≤40% (n = 66) | >40% (n = 145) | p Value | |
| Age (years) | 62.7 ± 15.1 | 56.5 ± 12.8 | 0.002 |
| Men | 52 (79%) | 119 (82%) | 0.573 |
| Race/ethnicity | 0.522 | ||
| White | 30 (46%) | 56 (39%) | |
| Black | 6 (9%) | 20 (14%) | |
| Asian | 22 (33%) | 41 (28%) | |
| Hispanic | 6 (9%) | 19 (13%) | |
| Other | 2 (3%) | 9 (6%) | |
| Diabetes mellitus | 15 (23%) | 28 (19%) | 0.568 |
| Hypertension | 29 (44%) | 66 (46%) | 0.831 |
| Coronary artery disease ⁎ | 17 (26%) | 24 (17%) | 0.117 |
| Dyslipidemia † | 17 (26%) | 38 (26%) | 0.945 |
| Tobacco use | 21 (32%) | 62 (43%) | 0.131 |
| Alcohol abuse | 4 (6%) | 17 (12%) | 0.238 |
| Systolic blood pressure (mm Hg) | 125 ± 40 | 139 ± 36 | 0.019 |
| Mechanical ventilation | 19 (29%) | 9 (6%) | <0.001 |
| Creatinine (mg/dl) | 1.22 ± 0.69 | 1.00 ± 0.34 | 0.002 |
| <1.0 | 26 (39%) | 93 (64%) | |
| 1.0–1.5 | 30 (45%) | 43 (30%) | |
| >1.5 | 10 (15%) | 7 (5%) | |
| Electrocardiographic characteristics | |||
| ST-segment elevation (mm) | 3.8 ± 3.4 | 2.1 ± 1.6 | <0.001 |
| Anterior ST-segment elevation | 39 (59%) | 39 (27%) | <0.001 |
| Inferior ST-segment elevation | 10 (15%) | 72 (50%) | <0.001 |
| Sinus rhythm | 48 (73%) | 127 (88%) | 0.008 |
| Culprit coronary artery | <0.001 | ||
| Left main or left anterior descending | 47 (71%) | 53 (37%) | |
| Left circumflex | 5 (8%) | 27 (19%) | |
| Right | 12 (18%) | 62 (43%) | |
| Other | 2 (3%) | 3 (2%) | |
| Percutaneous coronary intervention | 62 (94%) | 137 (94%) | 0.874 |
| Door-to-balloon time (min) | 83 (66–102) | 74 (62–112) | 0.220 |
⁎ Coronary artery disease was defined as a history of coronary artery disease as documented on the treating ED physician’s charted note.
† Dyslipidemia was defined as a history of dyslipidemia as documented on the treating ED physician’s charted note.
In the multivariate logistic regression model, a history of coronary artery disease, ST-segment elevation height on electrocardiography, the presence of dominant inferior ST-segment elevations, baseline creatinine, and the need for mechanical ventilation were significantly associated with the outcome of depressed LV function ( Table 2 ). The strongest predictor of normal LV function was inferior ST-segment elevations (adjusted odds ratio 0.12, 95% confidence interval 0.06 to 0.35 p <0.001), and the strongest predictor of depressed LV function was the need for mechanical ventilation in the ED (adjusted odds ratio 3.98, 95% confidence interval 1.41 to 11.19, p = 0.009). In adjusted analyses, age, gender, diabetes, and hypertension were not significantly associated with the outcome.
| Predictor | Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (per year) | 1.03 (1.01–1.06) | 0.003 | 1.02 (0.99–1.04) | 0.255 |
| Male gender | 0.81 (0.39–1.68) | 0.573 | 0.72 (0.28–1.84) | 0.490 |
| Diabetes mellitus | 1.23 (0.61–2.50) | 0.568 | 1.09 (0.41–2.86) | 0.868 |
| Hypertension | 0.94 (0.52–1.69) | 0.831 | 0.71 (0.33–1.53) | 0.381 |
| Coronary artery disease ⁎ | 1.75 (0.86–3.54) | 0.120 | 3.12 (1.26–7.71) | 0.014 |
| ST-segment elevation >2 mm on electrocardiography | 3.22 (1.76–5.89) | <0.001 | 2.78 (1.31–5.87) | 0.008 |
| Inferior ST-segment elevation | 0.18 (0.09–0.38) | <0.001 | 0.12 (0.05–0.31) | <0.001 |
| Creatinine (mg/dl) | ||||
| <1.0 | Reference | Reference | ||
| 1.0–1.5 | 2.55 (1.35–4.82) | 0.004 | 2.38 (1.08–5.26) | 0.032 |
| >1.5 | 5.22 (1.81–15.05) | 0.002 | 6.35 (1.66–24.31) | 0.007 |
| Mechanical ventilation | 6.11 (2.59–14.43) | <0.001 | 3.98 (1.41–11.19) | 0.009 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


