In patients treated with implantable cardioverter defibrillator (ICD), prediction of both overall survival and occurrence of shocks is important if improved patient selection is desired. We prospectively studied the predictive value of biomarkers and indexes of cardiac and renal function and spectral microvolt T-wave alternans testing and 24-hour Holter variables in a population who underwent first ICD implantation. Consecutive patients in sinus rhythm with ischemic or dilated cardiomyopathy scheduled for primary or secondary prophylactic ICD implantation were enrolled. Exercise microvolt T-wave alternans and 24-hour Holter for number of ventricular premature contractions (VPCs), deceleration capacity, heart rate variability, and heart rate turbulence were done. Death of any cause and first appropriate ICD shock were defined as end points. Over 33 ± 15 months of follow-up, 36 of 253 patients (14%) received appropriate shocks and 39 of 253 patients (15%) died. Only 3 of 253 patients (1%) died after receiving at least 1 appropriate shock. In univariate analyses, New York Heart Association class, ejection fraction, N-terminal pro brain-type natriuretic peptide (NT-proBNP), renal function, ICD indication, deceleration capacity, heart rate variability, and heart rate turbulence were predictive of all-cause mortality and VPC number and deceleration capacity predicted first appropriate shock. NT-proBNP (≥1,600 pg/ml) was identified as the only independent predictor of all-cause mortality (hazard ratio 3.0, confidence interval 1.3 to 7.3, p = 0.014). In contrast, VPC number predicted appropriate shocks (hazard ratio 2.3, confidence interval 1.0 to 5.5, p = 0.047) as the only independent risk marker. In conclusion, NT-proBNP is a strong independent predictor of mortality in a typical prospective cohort of newly implanted patients with ICD, among many electrocardiographic and clinical variables studied. Number of VPCs was identified as a predictor of appropriate shocks (clinicaltrials.gov: NCT02010515 ).
Implantable cardioverter defibrillators (ICDs) are recommended for the prevention of sudden cardiac death (SCD). However, a large number of patients never receive an appropriate shock from their device. Therefore, predictors for survival of patients with ICD in general and ICD shocks in specific need to be identified for improved patient selection. Microvolt T-wave alternans (MTWA) has been shown to improve the selection of primary prophylactic ICD candidates and has been recommended in sudden death risk stratification guidelines. Unfortunately, later trials have yielded equivocal findings. Other traditional electrocardiographic risk stratifiers, such as Holter variables, especially parameters of autonomic tone, and the signal-averaged electrocardiogram (SAECG) have never been tested in patients with ICD, although they have been used as inclusion criteria in large randomized ICD trials. We, therefore, prospectively studied a combination of selected risk stratifiers in a consecutive single-center ICD cohort featuring primary and secondary prophylactic indications. Only ischemic or nonischemic dilated cardiomyopathy patients were enrolled, and stable sinus rhythm was a major inclusion criteria. The prognostic value for all-cause mortality or first appropriate shock was evaluated for ventricular premature contractions (VPCs), nonsustained ventricular tachycardia, heart rate variability (HRV), heart rate turbulence and acceleration and deceleration capacity from Holter monitoring, exercise MTWA (Cambridge heart method) and clinical variables, such as N-terminal pro brain-type natriuretic peptide (NT-proBNP), renal function, cardiac disease, and ICD indication.
Methods
Consecutive patients who underwent first ICD or cardiac resynchronization therapy with defibrillator (CRT-D) implantation from 2008 to 2011 at our institution were eligible for this prospective observational study. All devices were implanted for approved primary and secondary prophylactic indications. Inclusion criteria were ischemic or dilated cardiomyopathy, sinus rhythm, and age ≥18 years. All patients gave their informed consent to the protocol. The study was conducted according to the Declaration of Helsinki and was registered (clinicaltrials.gov: NCT02010515 ). Patients not in sinus rhythm at baseline were excluded.
Prospectively, all patients underwent medical history, physical examination, and standard blood tests including serum creatinine for calculation of the estimated glomerular filtration rate using a constant c of 175 and NT-proBNP, a 12-lead electrocardiogram (ECG, MacVU 5500; GE, Milwaukee, Wisconsin), and echocardiography using the Simpson method for determination of left ventricular ejection fraction. Additionally, SAECG, exercise-based MTWA, and 24-hour Holter ECG were performed.
SAECG was recorded using a GE Marquette MAC 5000 (General Electric, Fairfield, Connecticut). In 30 of 253 patients (12%), an SAECG could not be recorded or was not analyzable for technical reasons.
MTWA testing was performed in 223 of 253 patients (88%) using a CH2000 station (Cambridge Heart, Tewksbury, Massachusetts) and heart rate elevation by means of exercise in 161 of 223 patients (72%). In patients unable to undergo exercise testing and scheduled for implantation of an atrial lead, testing was done after the ICD/CRT-D implantation by atrial (43 of 223, 19%) or biventricular pacing (19 of 223, 9%). It could not be completed in 30 patients because patients who underwent single-lead ICD implantation were unable to undergo ergometry. Beta blockers were not withheld as recommended. The test results were reviewed according to standard rules and graded by 2 independent and blinded investigators (in brief: sustained alternans ≥1.9 μV and alternans ratio >3 lasting at least 1 minute with an onset heart rate ≤110 beats/min: positive; maximum heart rate with noise ≤1.8 μV, rate of premature ventricular complexes <10% and without sustained alternans ≥105 beats/min [A-rules] or ≥80 beats/min with a difference to the maximum heart rate ≤5 beats/min [B-rules]: negative; otherwise: indeterminate). Positive and indeterminate results were grouped as non-negative results.
Dual-channel 24-hour Holter ECG was recorded using a digital portable recorder (Lifecard CF; Delmar Reynolds/Spacelabs Healthcare, Snowqualmie, Washington); 235 of 253 (93%) were accepted for statistical analysis; the remaining 18 patients had recordings of poor quality or a too short time period and were excluded from the final analysis. Holter analysis was done using semiautomatic software (Pathfinder, version 8.602; Delmar Reynolds/Spacelabs Healthcare). The number of VPC and the number of ventricular runs ≥3 beats (nonsustained ventricular tachycardia), each normalized for a recording time of 24 hours, were determined. HRV including standard deviation of RR intervals (SDNN), root mean square of successive differences in RR intervals, and frequency domain parameters (quotient of low frequency and high frequency) were calculated (HRV-Tools, version 1.74; Delmar Reynold/Spacelabs Healthcare). Heart rate turbulence (HRT) including turbulence onset and slope and acceleration and deceleration capacity were computed by means of open-source HRT software (Librasch Calc, 1.02 Schneider R and Schmidt G, TU Munich). Holter recordings with ≥30% atrial pacing (n = 20) were excluded from HRV, HRT, and deceleration capacity analysis for methodological reasons, HRT could not be determined in patients without suitable VPC (n = 19). Therefore, HRV, HRT, acceleration, and deceleration capacity were available in 210 of 235 (89%), 196 of 235 (83%), 209 of 235 (89%), and 210 of 235 (89%), respectively.
ICD or CRT-D device selection at implantation varied (Biotronik GmbH, Berlin, Germany; Medtronic Inc., Fridley, Minnesota; Boston Scientific, Natick, Massachusetts) according to patient needs and at the implanter’s discretion. The first programming was standardized: Two zones were programmed with the VT zone starting at 170 beats/min lasting 2.5 to 5 seconds or 16 to 24 beats and treated with antitachycardia pacing (ATP, standard: 2 × 3 programmed), followed by maximum output high-voltage shocks if recurrent ATP failed to terminate the arrhythmia. The VF zone started at 220 beats/min lasting for 1 to 2.5 seconds or 12 of 16 to 18 of 24 beats and was treated with maximum output high-voltage shocks (after ATP during charging if available). In case of a history of VT or induced sustained VTs, the average rate of clinical VTs prompted a VT zone at least 10 beats/min slower. Algorithms for improved detection of supraventricular arrhythmias (onset, stability, Biotronik “S.M.A.R.T.”, Medtronic “Wavelet” or “PRLogic”, Boston Scientific “RhythmID”) were activated. Single-chamber ICDs were programmed to ventricular demand pacing with 40 beats/min, dual-chamber ICD, and CRT-D to DDD 50 or 60 to 130 beats/min. Dual-chamber ICD had prolonged AV intervals set to reduce the percentage of RV pacing. Standard programming could be varied during follow-up according to individual patient’s needs.
Prospectively defined co-primary end points of the study were (1) the occurrence of a first appropriate ICD shock for malignant ventricular arrhythmia (as determined from the ICD’s EGM recordings) and (2) all-cause mortality. As we aimed to identify predictors of truly life-saving ICD therapies, successful or inappropriate ATP or inappropriate ICD shocks were not considered as end points. Data from our institution, other hospitals, the patients’ general practitioner, and local authorities were reviewed for assessment of all-cause mortality.
All statistical analyses were performed using SPSS, version 21.0 (SPSS Inc., Chicago, Illinois) and SPSS SamplePower, version 3. For the purpose of sample size calculation, annual mortality was estimated as 9% taking into account enrollment of more elderly patients with more co-morbidities. According to the Schoenfeld formula, a total of 36 events provides a power of 80% for a 2-sided test at the usual 5% significance level in the Cox proportional hazards regression as long as the hazard ratio (HR) is larger than 2.45 comparing with equally sized groups. All results are presented as mean ± SD for continuous variables and as frequencies (proportions) for categorical variables. Kaplan-Meier survival probability curves were computed to compare event rates in subgroups using the log-rank test. Univariate predictors with a p <0.1 were included in multivariate Cox proportional hazard models and stepwise backward elimination was performed. In addition, biomarkers and electrocardiographic predictors of all-cause mortality with a univariate p <0.1 were adjusted for clinical predictors with a p <0.1 to avoid that interrelated variables such as Holter parameters of autonomic tone (SDNN, quotient of low frequency and high frequency, HRT, and acceleration and deceleration capacity) are introduced to the Cox model simultaneously. Missing values were excluded from analysis (complete case analysis). Receiver operating characteristic (ROC) curves were used to quantitate test characteristics. Odds ratios (ORs) were calculated using contingency tables. For all tests, a p <0.05 was accepted for statistical significance.
Results
A total of 253 patients with ischemic or dilated cardiomyopathy (71% and 29%, respectively) were enrolled at first ICD implantation. ICD therapy was recommended for primary prophylaxis of SCD in 69% and for secondary prophylaxis in 31%, respectively; 43% of the latter had been successfully resuscitated from cardiac arrest due to VF. Patients were predominantly male (77%), with a mean age of 67 ± 11 years, mean ejection fraction was 30 ± 11%, 53% of patients presented with New York Heart Association functional class II symptoms, 44% with class III symptoms, and 3% with class I symptoms, respectively (of the latter, all had secondary prophylactic indication). NT-proBNP averaged 3,433 ± 6,023 pg/ml, and estimated glomerular filtration rate averaged 68.2 ± 24.5 ml/min/1.73 m 2 . Most patients received typical heart failure medications (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker 93%, beta-adrenergic receptor blockers 92%, loop diuretics 61%, and mineralocorticoid receptor antagonist 54%). Only few patients were treated with antiarrhythmic drugs (amiodarone 18% and flecainide 2%). A history of paroxysmal atrial fibrillation was known at enrollment in 29% of patients, 81% had arterial hypertension diagnosed, and 30% had diabetes mellitus, respectively. Patients were implanted with single-chamber ICDs in 39% and dual-chamber ICDs in 35%. The remaining 26% were given a device for cardiac resynchronization therapy (CRT-D).
Mean QRS duration was 125 ± 31 ms; 129 of 253 (51%) had a QRS duration <120 ms, typical left bundle branch block was present in 68 of 253 (27%) of the cohort. Mean QT interval was 418 ± 52 ms. SAECG revealed a mean filtered QRS duration of 149 ± 29 ms, a mean RMS voltage of 29 ± 68 μV, and a mean duration of low amplitude signals of 50 ± 28 ms. The MTWA test result was positive in 72 of 223 (32%), it was negative in 78 of 223 (35%) following A-rules and in 118 of 223 (53%) following B-rules, respectively. Indeterminate test results were found in 73 of 223 (33%) using A-rules and 33 of 223 (15%) using B-rules. Indeterminate test results (following B-rules) were caused by excessive ectopic beats in 15 of 33 (45%) of indeterminate MTWA patients, by chronotropic incompetence and a maximal heart rate <80 beats/min in 10 of 33 (30%) indeterminate patients, and poor technical quality or bad signal to noise ratio in 8 of 33 (25%) of all indeterminate tests, respectively. Mean heart rate on Holter monitoring was 69 ± 11 beats/min, mean number of VPC normalized to 24 hours was 2,356 ± 4,351; 38 of 235 patients (16%) had at least 1 ventricular run ≥3 beats. Mean SDNN was 90 ± 40 ms, root mean square of successive differences in RR intervals 23 ± 15 ms, and mean quotient of low frequency and high frequency 2.9 ± 3.9; mean turbulence onset from HRT was −0.1 ± 1.8%, mean turbulence slope 3.2 ± 4.1 ms/RR interval, mean acceleration capacity −8.2 ± 8.6 ms, and mean deceleration capacity 0.4 ± 7.3 ms.
Overall follow-up was 33 ± 15 months (range: 10 days, this patient died due to progressive heart failure early after ICD implantation, to 61 months). Appropriate ICD shocks occurred in 36 of 253 patients (14%). In 18 events, shocks were delivered for malignant ventricular arrhythmia in the VF zone (at a mean cycle length of 235 ± 22 ms). Another 18 shock events occurred in the VT zone following failed anti-tachycardia pacing for an index VT with a mean cycle length of 302 ± 17 ms. Thirty-nine of 253 patients (15%) died during FU, only 3 of 253 (1%) received an appropriate ICD shock before death.
Kaplan-Meier analysis showed that VPC frequency on Holter (median 525 beats/24 hours; p = 0.01; Figure 1 ) and deceleration capacity from Holter (median 2.2 ms; p = 0.03; Figure 1 ) were associated with a higher probability of ICD shock.
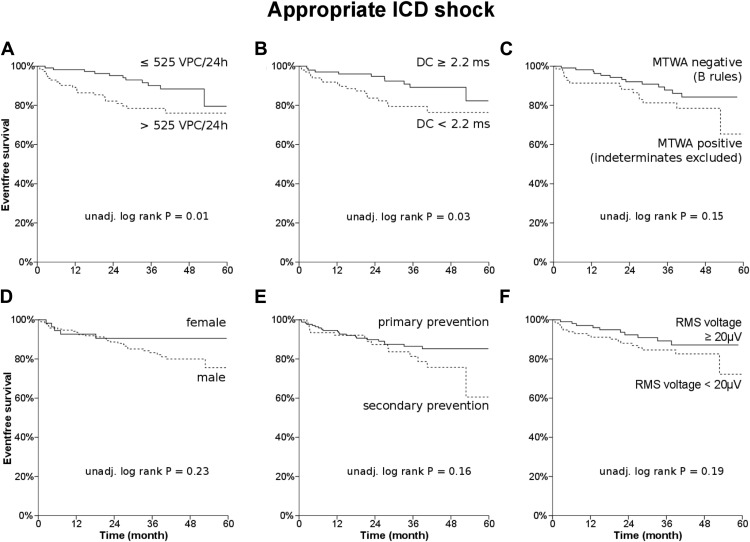
MTWA did not reach statistical significance when assessing its association with ICD shocks (non-negative vs negative; p = 0.20). When indeterminate MTWA tests were omitted from the non-negative group, the comparison still did not reach statistical significance (positive vs negative following B-rules, p = 0.15; Figure 1 ). Negative MTWA (vs non-negative) following B-rules had a sensitivity and a specificity of 58% and 55% (negative/positive predictive value 89%/17%; OR 1.7; 95% confidence interval [CI] 0.8 to 3.6; p = 0.25) to predict first appropriate shock.
No statistically significant difference for the probability of ICD shocks was found in male gender (p = 0.23; Figure 1 ), secondary prophylactic ICD indication (p = 0.16; Figure 1 ), depressed RMS voltage from SAECG (median 19 μV; p = 0.19; Figure 1 ), occurrence of nonsustained ventricular tachycardia during Holter monitoring (p = 0.37), ejection fraction (median 30%, p = 0.77), NT-proBNP (median 1,599 pg/ml; p = 0.70), estimated glomerular filtration rate (median 66 ml/kg/1.73 m 2 ; p = 0.29), and HRT (onset median 0.1%, p = 1.00; slope median 1.9 ms/RRi, p = 0.86), respectively.
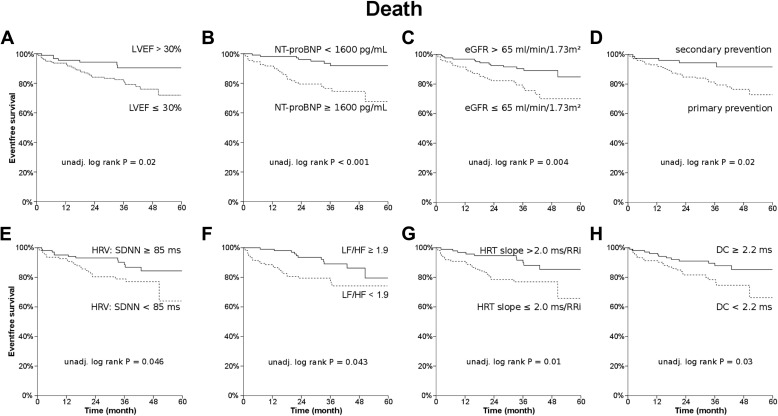
Kaplan-Meier analysis revealed a statistically significant association of all-cause mortality and the following variables: New York Heart Association functional class (I/II vs III; p = 0.03), ejection fraction (p = 0.02; Figure 2 ), NT-proBNP (p = 0.0002; Figure 2 ), estimated glomerular filtration rate (p = 0.004; Figure 2 ), ICD indication (p = 0.02; Figure 2 ), SDNN (median 84 ms; p = 0.046; Figure 2 ), quotient of low frequency and high frequency (median 1.9, p = 0.043; Figure 2 ), HRT slope (p = 0.01; Figure 2 ), and deceleration capacity (p = 0.03; Figure 2 ), respectively. A nonsignificant trend was found for age (p = 0.07) and VPC (p = 0.08), respectively. When both HRT variables were grouped using dichotomization by median, a nonsignificant trend was found (onset ≤0.1% and/or slope ≥2 ms/RRi vs onset >0.1% and slope <2 ms/RRi; p = 0.06). No statistically significant differences were found for ischemic cardiomyopathy (p = 0.18), MTWA (negative vs non-negative; p = 0.16), gender (p = 0.34), nonsustained ventricular tachycardia during Holter monitoring (p = 0.42), and HRT onset (p = 0.37), respectively. Negative MTWA (vs non-negative) following B-rules had a sensitivity and a specificity of 59% and 55% (negative/positive predictive value 88%/19%; OR 1.7; 95% CI 0.8 to 3.7; p = 0.19) to predict all-cause mortality. In addition, univariate Cox regression was performed, and the results for prediction of first appropriate shock and all-cause mortality are given in Tables 1 and 2 (left columns). ROC analysis was performed to determine the diagnostic utility of the variables for the prediction of appropriate ICD shocks and of all-cause mortality, respectively ( Tables 1 and 2 , right columns). NT-proBNP showed the largest area under the curve (p = 0.000003; Figure 3 ).
| Variable | APPROPRIATE SHOCK | ||||
|---|---|---|---|---|---|
| N | Univariate | ROC | |||
| HR (95% CI) | P | AUC (95% CI) | P | ||
| Men | 253 (100%) | 1.8 (0.7-4.6) | 0.24 | ||
| Age (≥ 70 years | 253 (100%) | 1.0 (0.5-1.9) | 0.99 | 0.48 (0.4-0.57) | 0.76 |
| NYHA functional class III | 253 (100%) | 1.4 (0.7-2.8) | 0.38 | ||
| left-ventricular ejection fraction (≤ 30%) | 253 (100%) | 0.9 (0.5-1.8) | 0.77 | 0.49 (0.38-0.59) | 0.79 |
| NT-proBNP (≥ 1600 pg/mL) | 223 (88%) | 1.2 (0.6-2.4) | 0.70 | 0.47 (0.35-0.59) | 0.61 |
| glomerular filtration rate (≤ 65 mL/min/1.73m 2 ) | 253 (100%) | 0.7 (0.4-1.4) | 0.29 | 0.42 (0.32-0.52) | 0.12 |
| ICD indication (secondary prophyl.) | 253 (100%) | 1.6 (0.8-3.1) | 0.17 | ||
| Ischemic cardiomyopathy | 253 (100%) | 1.1 (0.5-2.3) | 0.79 | ||
| ECG and SAECG | |||||
| QRS (≥ 120 ms) | 253 (100%) | 0.8 (0.4-1.5) | 0.44 | 0.46 (0.35-0.56) | 0.40 |
| filtered QRS duration (> 145 ms) | 223 (88%) | 0.9 (0.4-1.9) | 0.84 | 0.52 (0.41-0.64) | 0.70 |
| RMS voltage (< 20 μV) | 223 (88%) | 1.7 (0.8-3.6) | 0.20 | 0.61 (0.49-0.74) | 0.05 |
| Low-amplitude signals (≥ 45 ms) | 223 (88%) | 1.2 (0.6-2.6) | 0.58 | 0.55 (0.44-0.67) | 0.36 |
| T wave alternans | |||||
| MTWA: non-negative vs. negative ∗ | 223 (88%) | 1.6 (0.8-3.2) | 0.20 | ||
| MTWA: positive vs. negative | 190 (75%) | 1.7 (0.8-3.7) | 0.16 | ||
| Holter | |||||
| VPC (> 525 beats/24h) | 235 (93%) | 2.4 (1.2-5.0) | 0.02 | 0.6 (0.48-0.71) | 0.07 |
| Deceleration capacity (< 2.2 ms) | 210 (83%) | 2.4 (1.1-5.1) | 0.03 | 0.6 (0.49-0.71) | 0.08 |
| Acceleration capacity (< –6.0 ms) | 209 (83%) | 2.5 (1.1-5.8) | 0.03 | 0.68 (0.58-0.77) | 0.003 |
| HRV: SDNN (< 85 ms) | 210 (83%) | 0.6 (0.3-1.4) | 0.25 | 0.41 (0.31-0.51) | 0.11 |
| HRV: low frequency/high frequency (< 1.9) | 202 (80%) | 1.2 (0.5-2.5) | 0.67 | 0.47 (0.35-0.58) | 0.61 |
| HRT: turbulence onset (> 0.1%) | 196 (77%) | 1.0 (0.5-2.1) | 1.00 | 0.46 (0.34-0.59) | 0.53 |
| HRT: turbulence slope (≤2.0 ms/RRi) | 196 (77%) | 0.9 (0.4-2.0) | 0.86 | 0.48 (0.37-0.58) | 0.68 |
∗ B rules; using A rules, HR was 1.4 (95% CI, 0.7 to 3.1; p = 0.38).
| Variable | ALL-CAUSE MORTALITY | ||||
|---|---|---|---|---|---|
| N | Univariate | ROC | |||
| HR (95% CI) | P | AUC (95% CI) | P | ||
| Men | 253 (100%) | 0.7 (0.4-1.4) | 0.35 | ||
| Age (≥ 70 years | 253 (100%) | 1.8 (0.9-3.4) | 0.08 | 0.59 (0.5-0.68) | 0.08 |
| NYHA functional class III | 253 (100%) | 2.3 (1.1-5.0) | 0.04 | ||
| left-ventricular ejection fraction (≤ 30%) | 253 (100%) | 2.6 (1.1-5.9) | 0.02 | 0.66 (0.58-0.75) | 0.001 |
| NT-proBNP (≥ 1600 pg/mL) | 223 (88%) | 4.3 (1.8-9.9) | <0.001 | 0.76 (0.67-0.84) | <0.001 |
| glomerular filtration rate (≤ 65 mL/min/1.73m 2 ) | 253 (100%) | 2.6 (1.3-5.0) | 0.01 | 0.66 (0.57-0.75) | 0.001 |
| ICD indication (primary prophyl.) | 253 (100%) | 3.0 (1.2-7.8) | 0.02 | ||
| Ischemic cardiomyopathy | 253 (100%) | 1.7 (0.8-3.7) | 0.19 | ||
| ECG and SAECG | |||||
| QRS (≥ 120 ms) | 253 (100%) | 0.9 (0.5-1.8) | 0.87 | 0.51 (0.41-0.61) | 0.84 |
| filtered QRS duration (> 145 ms) | 223 (88%) | 1.2 (0.6-2.4) | 0.65 | 0.51 (0.39-0.62) | 0.91 |
| RMS voltage (< 20 μV) | 223 (88%) | 1.0 (0.5-2.0) | 0.99 | 0.47 (0.37-0.58) | 0.62 |
| Low-amplitude signals (≥ 45 ms) | 223 (88%) | 1.0 (0.5-2.1) | 0.93 | 0.47 (0.37-0.57) | 0.62 |
| T wave alternans | |||||
| MTWA: non-negative vs. negative ∗ | 223 (88%) | 1.6 (0.8-3.2) | 0.16 | ||
| MTWA: positive vs. negative | 190 (75%) | 1.6 (0.8-3.4) | 0.21 | ||
| Holter | |||||
| VPC (> 525 beats/24h) | 235 (93%) | 1.8 (0.9-3.4) | 0.08 | 0.58 (0.49-0.67) | 0.11 |
| Deceleration capacity (< 2.2 ms) | 210 (83%) | 2.2 (1.1-4.4) | 0.03 | 0.61 (0.52-0.7) | 0.04 |
| Acceleration capacity (< –6 ms) | 209 (83%) | 1.3 (0.7-2.5) | 0.45 | 0.51 (0.41-0.61) | 0.92 |
| HRV: SDNN (< 85 ms) | 210 (83%) | 2.0 (1.0-4.2) | 0.05 | 0.58 (0.48-0.67) | 0.16 |
| HRV: low frequency/high frequency (< 1.9) | 202 (80%) | 2.1 (1.0-4.4) | 0.05 | 0.57 (0.46-0.68) | 0.24 |
| HRT: turbulence onset (> 0.1%) | 196 (77%) | 1.4 (0.7-2.8) | 0.37 | 0.57 (0.48-0.67) | 0.21 |
| HRT: turbulence slope (≤2.0 ms/RRi) | 196 (77%) | 2.5 (1.2-5.3) | 0.02 | 0.68 (0.58-0.78) | 0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


