Pre-operative Algorithms to Optimize Autogenous Access
Martin R. Back
Based upon the widely held assumption that the patency rates for autogenous hemodialysis accesses are superior to their prosthetic counterparts, the National Kidney Foundation Dialysis Outcomes Quality Initiative Clinical Practice Guidelines for Vascular Access (DOQI) have recommended that ≥50% of all new permanent hemodialysis accesses be autogenous with an overall prevalence of 40%. Despite the DOQI’s aim at improving outcomes in patients with end-stage renal disease, the prevalence of autogenous accesses in the United States remains well below this target (24%) and is significantly lower than in Europe (80%), as reported from the large, prospective Dialysis Outcomes and Practice Patterns Study (DOPPS).
The adequacy of the arterial inflow and the forearm cephalic vein for constructing an autogenous access has traditionally been determined by physical examination in conjunction with a tourniquet. However, these simple strategies frequently fail to identify all possible artery/vein combinations amenable to an autogenous access. This process is further complicated by our aging dialysis population, with its associated comorbidities and prior venipuncture/access procedures. Additionally, other important anatomic features, including arterial diameter, distribution of the calcium within the arterial wall, size/extent of the upper arm veins (i.e., basilic, cephalic), quality of the vein lumen (i.e., presence of fibrosis), and the patency of the central veins are not apparent on physical examination alone. Pre-operative strategies to improve operative planning for autogenous access, thereby overcoming the limitations of physical examination, have centered on use of noninvasive duplex ultrasound and/or contrast arteriography/venography. The use of these imaging techniques has paralleled the expanding use of creative venous transposition techniques with the basilic and forearm veins to expand the autogenous options beyond the direct configurations (i.e., radiocephalic or brachiocephalic).
Pre-operative Imaging Techniques
Noninvasive Testing
Both the routine and selective use of preoperative duplex ultrasound for planning hemodialysis access have been associated with an increase in the use of autogenous accesses relative to the various institutional experiences pre-DOQI. Although the anatomic criteria for defining a suitable vein conduit and preferences for access location/configuration vary somewhat between reports, the techniques of noninvasive testing and imaging remain similar. Our protocol was developed at the University of South Florida in 1998 and is described below. In addition to the existing CPT code (93990) for duplex imaging of functioning prosthetic and autogenous accesses, a new code (G0365) has been created in 2005 for pre-operative vessel (arterial and venous) mapping before first-time construction of a fistula in a limb without prior access creation. Consistency of reimbursement for use of this code remains to be determined.
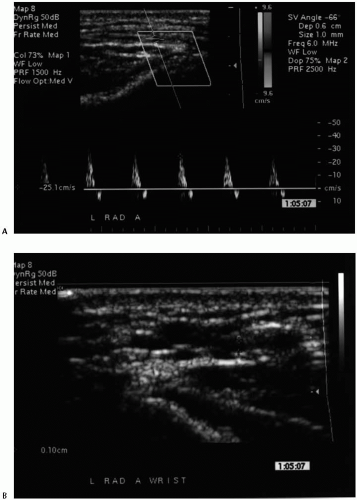
The noninvasive testing should be performed in a warm room and begins with an upper-extremity arterial study using continuous wave Doppler ultrasound and selected blood pressure measurements. Notably, the nondominant extremity is used preferentially due to the small but real potential to compromise the dominant hand by the ultimate access procedure. Brachial artery pressures are measured and the corresponding velocity waveforms at the brachial (antecubital), radial (wrist), and ulnar (wrist) arteries recorded. Formal blood pressure measurements in the radial and ulnar arteries are not obtained due to the frequent presence of calcification within the vessel wall that precludes accurate assessment. A photoplethysmographic (PPG) waveform and pressure are recorded for the third digit using the appropriate sensor and a digital cuff, respectively. A pressure gradient of ≥15 mmHg between the brachial arteries and/or the absence of a triphasic velocity waveform (at the brachial artery) suggest a hemodynamically significant inflow stenosis. Similarly, a pressure gradient of ≥30 mmHg between the ipsilateral brachial artery and the third digit and/or a diminution in the corresponding velocity waveforms suggests the presence of significant occlusive disease distal to the antecubital fossa (outflow). An Allen test is performed to assess the relative contributions of the radial and ulnar artery to the digital/palmar perfusion using the digital PPG waveform. Further noninvasive testing beyond this point is only performed if there is no evidence of hemodynamically significant arterial occlusive disease in the inflow/outflow vessels and the absolute digital pressures exceed 80 mmHg. Notably, these strict criteria were imposed in an attempt to reduce the incidence of postoperative hand ischemia or the “steal” phenomenon. Arterial testing is completed with transverse imaging of the
distal radial and brachial arteries using a high-resolution duplex scanner (Philips/ATL HDI 3000 or 5000, Bothell, WA) and L 7-4 MHz or CL 10-5 MHz transducer probe. An arterial diameter of ≥2.0 mm without extensive calcification is considered adequate as the inflow site for the access (Fig. 83-1).
distal radial and brachial arteries using a high-resolution duplex scanner (Philips/ATL HDI 3000 or 5000, Bothell, WA) and L 7-4 MHz or CL 10-5 MHz transducer probe. An arterial diameter of ≥2.0 mm without extensive calcification is considered adequate as the inflow site for the access (Fig. 83-1).
Venous duplex imaging of the central veins is then performed on the arm deemed suitable for an autogenous access based upon the arterial studies. The patient is positioned supine on a stretcher with head elevated while the arm is extended and placed on a pillow. The subclavian, brachial, and axillary veins are evaluated for both acute and chronic venous obstruction using color duplex imaging (L 7-4 MHz probe) and the standard augmentation maneuvers. The internal jugular vein is examined in patients with a previous, ipsilateral dialysis access catheter and those with abnormal studies in the axially/subclavian vein. The contralateral upper extremity is examined in patients with a suspected central venous stenosis (Figs. 83-2 and 83-3).
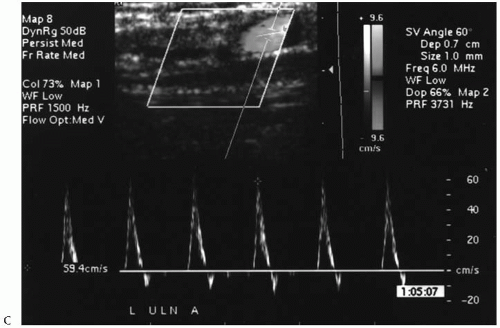
The superficial veins (cephalic and basilic) of the forearm are examined using a CL 10-5 MHz transducer in the extremity deemed acceptable based upon the arterial and central venous studies. Starting at the wrist and moving cephalad, the veins in the forearm are evaluated at multiple sites (usually 3 to 4 different sites). The examination includes determination of the diameter (outer wall to outer wall on transverse imaging) and depth beneath the skin, in addition to an assessment of compressibility and quality (wall thickening and fibrosis). Wrapping the extremity in a warm towel, having the patient perform repetitive hand exercises, and/or applying a tourniquet below the antecubital fossa, may augment the venous distention and facilitate identification of the veins that lie deep to the skin. Vein diameters of ≥2.5 mm are considered adequate for autogenous access construction. The segments of the cephalic and basilic veins spanning the antecubital fossa are then examined for the forearm veins that are deemed adequate. This occasionally necessitates moving the tourniquet more proximal on the arm. The superficial veins in the arm or upper arm (i.e., cephalic to the deltopectoral groove, basilic to its axillary confluence) are similarly examined if the forearm veins are <2.5 mm. If no suitable superficial vein is identified in the nondominant arm, then the contralateral arm is studied, presuming the results of the arterial and central vein studies are acceptable (Figs. 83-4 and 83-5). A summary of our noninvasive arterial and venous criteria for selecting suitable locations for autogenous accesses is shown in Table 83-1.
Contrast Arteriography/Venography
Invasive imaging may further help to plan the procedure and serves to confirm the choice based upon the noninvasive imaging. Most authors recommend selective upper-extremity arteriography and/or venography for the abnormalities found during noninvasive duplex examination, while others advocate a more liberal use of venography. However, standard, iodinated contrast is nephrotoxic and can potentially hasten the need for dialysis among those patients not yet actively dialyzing. Use of alternative contrast media, such as carbon dioxide for venography or gadolinium for arteriography, may avoid this potential risk of dye-associated renal injury. The other potential complications associated with invasive imaging include access vessel thrombosis, hemorrhage, pseudoaneurysm formation, distal embolization, contrast-induced venous thrombosis, and stroke resulting from the catheter manipulations within the aortic arch and supraaortic branches.
Pre-operative arteriography is recommended for patients with evidence of significant inflow occlusive disease (as detected by the noninvasive imaging) proximal to the planned anastomotic site. This usually occurs in patients with limited access options in the upper extremity, because alternative options in the contralateral extremity are routinely explored first. In the best-case scenario, a lesion amenable to balloon angioplasty/stenting is identified and definitively treated, thereby correcting the inflow lesion and permitting an autogenous access to be constructed at a later date. In a recent patient with a relative contraindication for a hemodialysis access in the contralateral extremity (lymphedema after prior mastectomy and axillary radiation), a focal subclavian stenosis was treated by angioplasty/stenting, thereby facilitating a staged autogenous radiocephalic access. Arteriography can also be used to assess the severity of forearm occlusive disease detected by noninvasive testing, to help estimate the likelihood of developing hand ischemia from the “steal syndrome,” and to help plan an arterial bypass in conjunction with a hemodialysis access. Indeed, we have performed several upper-extremity bypass procedures in patients with short segment (<15 cm) occlusions of the proximal forearm vessels (brachial, radial, or ulnar) to allow the construction of a brachial artery based on autogenous access. The final configurations were similar to the DRIL (distal revascularization interval ligation) procedure used for treating ischemic hands, although it was not necessary to “ligate” the intervening artery because it was already occluded. In general, preoperative arteriography is not usually necessary given our algorithm, although it does have wider applications for evaluating the nonmaturing autogenous access and for patients with postoperative hand ischemia.
Table 83-1 University of South Florida Noninvasive Criteria for Autogenous Hemodialysis Access | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
The arch and upper-extremity arteriograms are best performed using the standard retrograde femoral approach with selective catheter placement in the subclavian artery to limit the requisite contrast volume for the runoff imaging. The distal forearm and hand are usually imaged immediately after the aortic arch injection, because these distal vessels can go into spasm after the contrast injection, thereby reducing the quality of the images. Endovascular treatment (balloon angioplasty/stenting) of innominate, subclavian, axillary, and proximal brachial arteries can be treated using a retrograde brachial approach. Indeed, this is likely the optimal approach, given its direct approach and proximity. Dual access through the femoral and brachial arteries is often helpful in this setting, with the former approach used for the diagnostic procedure and the latter for the therapeutic intervention. The technical and hemodynamic success of all peripheral interventions should be confirmed using duplex imaging and standard pressure measurements prior to constructing the autogenous access.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree