Chapter 15 Postthrombotic Syndrome
Historical Background
Postthrombotic syndrome (PTS) remains an important health care problem in United States. A population-based study showed that the incidence of venous ulcers currently approaches 18 per 100,000 habitants per year.1 The same study identified that PTS is responsible for economic expenses estimated at least $200 million.1
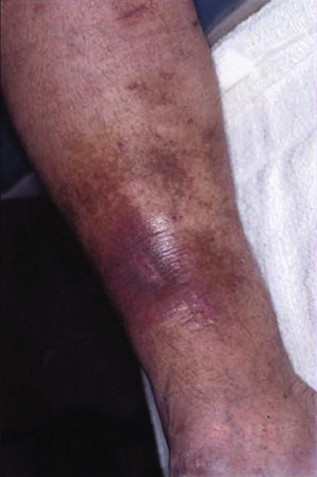
Diagnosis of PTS is based on history and clinical presentation. The syndrome consists of signs and symptoms of heaviness, intolerance to exercises, pain, leg edema, paresthesia, cramps, pruritus that may evolve to skin damage such as hyperpigmentation, lipodermatosclerosis, and ulcers (Fig. 15-1). The severity of PTS is measured by different scales and scores assigning points for the presence of each sign and symptom. Although there is a good association with PTS severity, further refinement and validation are necessary.2–5
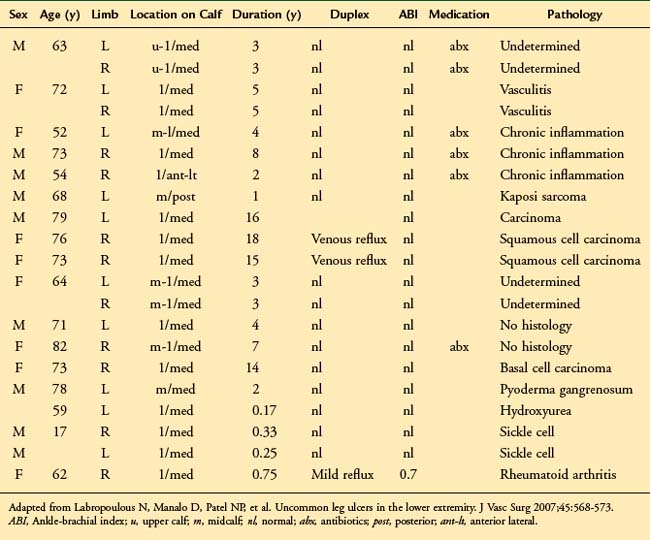
Attention to differential diagnosis should be outlined. Trauma, congenital venous disease (i.e., venous malformations, valve aplasia) and other causes of ulcerative disease such as rheumatologic (lupus, scleroderma, rheumatoid arthritis), oncologic (squamous and basal cell carcinoma), or infectious disorders (syphilis, lymphangitis) are potential causes of misdiagnosis and inappropriate treatment.6,7 Table 15-1 shows the location and cause of non–venous-related ulcers.
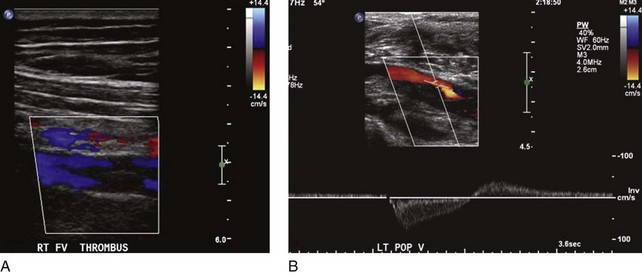
Not all patients who have had a documented episode of DVT sustain PTS. Recovery with no signs or symptoms of PTS occurs in two-thirds of the patients, and only the rest will eventually develop PTS with variable spectrum and severity.8 It appears that other factors are involved in the disease process, rendering some patients more vulnerable than others to PTS changes. Predisposing factors for PTS are age, sex, hypercoagulable state, obesity, immobility, and, most important, ipsilateral recurrent DVT. The latter is deemed to be the strongest factor for PTS, as several prospective studies have shown high odds for skin damage.9,10 Patients who had unprovoked DVT and are older than 65 years have a higher risk of recurrent DVT and therefore PTS.11 Residual thrombus was also reported as a cause of recurrent DVT12 (Fig. 15-2). In addition, patients who had DVT in more than one site, popliteal valve insufficiency, or a calf DVT associated with a proximal DVT also have an increased risk of recurrent DVT.13–15 Notably, the recurrence of DVT is likely to affect the proximal veins and is related to inadequate duration of anticoagulation.9 In a recent review, the limitations of studies on recurrent DVT are discussed.16 Most of the studies used a nonstandardized ultrasound analysis, giving limited or inaccurate information on the incidence of fatal pulmonary embolism (PE) as well as having poor documentation of anticoagulation and its monitoring. The effects of thrombolytic therapy and its socioeconomic and quality of life impact are also questions that remain unanswered.16 These are important because ipsilateral recurrent DVT has a great impact on the development of PTS.
Biomarkers have been used to estimate the odds of developing PTS. In a recent study of 305 patients with PTS, persistent elevated levels of D-dimer in the course of DVT were investigated. Patients with elevated levels of D-dimer at 4 months following DVT after stopping anticoagulation showed a fourfold risk of PTS.17
Progression of secondary cardiovascular disease (CVD) evolving to PTS has been demonstrated. A study of 73 limbs with secondary CVD showed overall progression in clinical CEAP classes in 31% of the limbs. Skin damage (C4-C6) rate strikingly rose from 4% at the first year to 25% in the 5-year follow-up. Other studies also investigated secondary CVD and clinical course of PTS.12 In an Italian cohort of 355 patients, the incidence of PTS increased from 17% after the first year to 29% at 8 years of follow-up.12 Development of reflux alone has been associated with PTS.10–17 However, a combination of reflux and obstruction is associated with more severe PTS than reflux or obstruction alone.10
Patients with a hypercoagulable state such as carriers of factor V Lieden (FVL), prothrombin gene mutation, or proteins C and S have been investigated for occurrence of PTS. In a recent study, 667 patients with DVT who sustained hypercoagulable state demonstrated no association with increased risk of PTS. Furthermore, heterozygosis for FVL was even less associated with PTS than in noncarriers.18 Another study of 387 patients tested for thrombophilia found no increased risk of PTS in carriers of FVL or prothrombin gene mutation.15 The same study also showed that the intensity of persistent signs and symptoms in the first month after the episode of acute DVT predicted the incidence of PTS in a dose-dependent manner in the first 2 years.15
Nonoperative Treatment
PTS generates high expenses for government and insurance companies due to disability premiums, loss of labor force, and need for rehabilitation and wound care. Thus, prevention should be always contemplated to minimize the socioeconomic impact of the disease. DVT prophylaxis is mandatory in different settings such as major surgeries, critically ill patients, and those with significant risk factors for DVT.19
Multiple reports have shown that elastic compression applied in patients with acute DVT may decrease the incidence of PTS by 50% at long term.20,21 This was evident in two review papers that analyzed all the relevant prospective studies.22,23 However, most of these trials had an inadequate sample size to resolve this conclusively. Currently, there is an ongoing randomized multicentre trial (SOX trial) that will provide data on the effectiveness of compression stockings on the prevention and severity of PTS, VTE recurrence, quality of life, and cost-effectiveness.24 It will also prospectively evaluate the predictive role of biomarkers in the development of PTS.
In patients with established PTS, a few treatments are available. Initial treatment for all patients with PTS is nonoperative, with compression to the legs in different levels and intensity. The use of compression stockings in 387 patients could not predict the progression or lack of PTS. However, patients who did use compression stockings had a risk of 20% of developing PTS compared with 47% who did not use compression stockings.12 The Cochrane collaboration review of 39 randomized control trials concluded that compression was more effective than no compression on ulcer healing.25 It was also shown that multicomponent systems were more effective in ulcer healing compared with single-component systems.
The shortcoming of compression therapy is that up to 20% of patients are noncompliant, and compliance with stockings does not change regardless of the symptoms of PTS.26 Complementary to the compression therapy, wound care is important to improve ulcer healing rates and patients’ quality of life. Many techniques have been applied in different settings varying from local care to more extensive surgical debridement.
More recently, in a small prospective randomized study of patients with PTS, exercise training for 6 months was associated with improvement in quality of life and improvement in scores on the Villalta scale.27 The authors concluded that these results should be confirmed in a large prospective randomized trial.
Interventional Treatment
Surgical and endovascular procedures are indicated when deep venous reflux or obstruction is present with or without superficial vein involvement in patients with skin damage or disabling symptoms. Prior to the endovenous techniques, obstruction used to be treated with bypass grafts. More recently, chronic venous obstruction is treated with balloon angioplasty and stenting. Bypass grafting is reserved for cases in which the endovenous approach is unsuccessful. However, the endovenous treatment for chronic venous obstruction is discussed extensively in Chapter 14. This chapter concentrates on the bypass grafting.
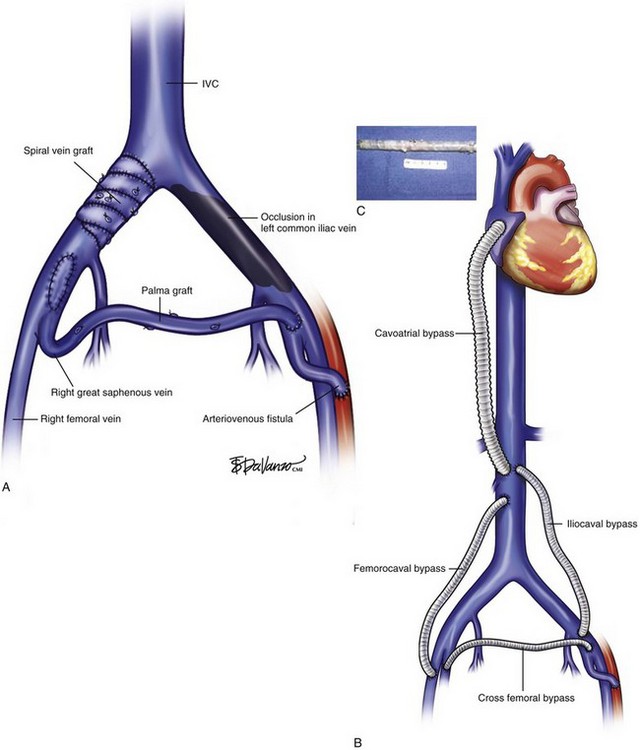
The treatment of proximal occlusion, including iliocaval segment, differs from that for femoropopliteal thrombotic occlusive disease. The higher velocity, significant blood volume, and better outflow of common femoral, iliac veins, and inferior vena cava (IVC) create a more amenable setting for treatment with either vein or prosthetic bypass grafts. A study of 44 venous reconstructions for nonmalignant iliofemoral and IVC occlusion was conducted at the Mayo Clinic.28 A wide variety of possible options for IVC and iliofemoral segment reconstruction were used, including great saphenous vein (GSV) crossover grafts (Palma procedure), supported expanded polytetrafluoroethylene (ePTFE), spiral vein grafts, and femoral vein patch angioplasty (Fig. 15-3). The overall primary and secondary patency at 3 years was 54% and 62%, respectively. Conversely, lower primary and secondary patency rates were found for iliocaval and femorocaval bypasses when analyzed separately, reaching only 38% and 54% at 2-year follow-up. The lower patency found in those bypasses was deemed to be related to the ePTFE bypass grafts. A small number of procedures failed to show statistical significance, although a trend toward higher patency rates was shown for GSV bypass grafts. Highest patency was also achieved with GSV crossover grafts, reaching 77% in a 4-year period, similar to other reports in the literature.28,29
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree