Fibrinolysis has not been used for the treatment of ST-segment elevation myocardial infarction (STEMI) in Denmark since 2005. We aimed to assess the positive predictive value of clinically suspected STEMI among consecutive patients in a real-world setting where all patients with suspected STEMI undergo acute coronary angiography. We evaluated the clinical diagnosis of consecutive patients with suspected STEMI admitted to Aarhus University Hospital between September 1, 2010, and August 31, 2011. Conclusive STEMI was defined as a patient with an identifiable culprit lesion by angiography. Of 615 patients with suspected STEMI, 483 (79%) had conclusive STEMI, and 132 (21%) did not have an identifiable culprit lesion. A higher proportion of patients with conclusive STEMI were men, whereas patients without conclusive STEMI were more likely to have diabetes mellitus (16% vs 10%; p = 0.04), left bundle branch block (24% vs 2%; p <0.001), hypertension (48% vs 36%; p = 0.01), or a history of coronary artery bypass surgery (8% vs 2%; p = 0.001). Compared with the overall 79% with conclusive STEMI, patients with left bundle branch block or a history of coronary artery bypass surgery had positive predictive values of only 26% and 41%, respectively. Our findings thus indicate that a substantial number of patients would have received fibrinolysis, without any potential benefit but with the inherent risk of bleeding complications, if acute angiography had not been an option.
In Denmark, primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI) has been the preferred reperfusion strategy after publication of the Danish Trial of Acute Myocardial Infarction-2 (DANAMI-2), and fibrinolysis as treatment for STEMI has not been used since 2005. The diagnosis is always made by physicians, and all patients with suspected STEMI in our catchment area are transported to our center for acute coronary catheterization. This offers a unique possibility to investigate the positive predictive value of physician-assessed suspected STEMI based on symptoms and electrocardiogram (ECG).
Methods
The study was conducted at Aarhus University Hospital, Skejby, Denmark, a tertiary cardiovascular center with referral relationships with 6 community hospitals. The total catchment area is a population of approximately 1,250,000 persons. Since 1997, Aarhus University Hospital has offered fully staffed cardiac catheterization services with an experienced interventional cardiologist service 24 hours a day, 7 days a week.
The study population consisted of consecutive patients with suspected STEMI, with ST-segment elevation in ≥2 consecutive leads of ≥0.2 mV in leads V 2 to V 3 or ≥0.1 mV in other leads, ST-depression ≥0.1 mV in V 1 to V 3 indicative of posterior STEMI, or presumed new left bundle branch block admitted for acute cardiac catheterization in Aarhus University Hospital between September 1, 2010, and August 31, 2011. The decision to activate the catheterization laboratory was made exclusively by the on-call cardiologist based on standard clinical and the foregoing ECG criteria. In all cases, ECGs were either transmitted wirelessly as part of routine prehospital patient care (with additional telephone interview), or the ECG was faxed and discussed on the telephone with the on-call medical doctor at the referral hospital. Patients with suspected STEMI were pretreated with 600 mg clopidogrel, 300 mg aspirin and 10,000 units heparin i.v., and were received directly in the catheterization laboratory. In a typical transport from the prehospital setting or transfer from a referral hospital, the on-call cardiologist awaited the arrival of the patient at the ambulance entrance. The on-call cardiologist accompanied the emergency medical service personnel from the ambulance directly to the catheterization laboratory. The emergency medical service personnel brought the patient on the gurney directly into the laboratory and assisted with transfer onto the radiography table. The on-call cardiologist and the PCI operator reviewed the ECG and the clinical information and performed necessary elements of a physical examination while the laboratory staff prepared the patient for the acute angiography. A bedside ECG was performed if relevant for the diagnosis. Door-to-balloon time was 26 minutes on average for patients transported from a referral hospital and 33 minutes for patients with field triage and transport directly to our center. After catheterization, patients were transferred to our acute coronary care unit. Except from the minority of patients with residence in our local catchment area, all clinically stable patients were transferred to referral hospitals within 24 hours.
An echocardiographic evaluation of the left ventricular function was typically performed in the acute coronary care unit shortly after cardiac catheterization and was performed in 550 (89%) of 615 patients.
Troponin T was measured on arrival in catheterization laboratory and again after 6 to 8 hours and 12 to 16 hours. Some of the referral hospitals used troponin I instead of troponin T. Online access to all measurements was available for all hospitals in the catchment area. Elevated cardiac marker levels were defined as a troponin T peak of ≥50 ng/L or a troponin I ≥0.04 μ/L.
For the purpose of this study, STEMI patients in whom a culprit coronary lesion was identified during cardiac catheterization were considered “conclusive STEMI.” The lesion was identified as culprit if there was a flow-limiting stenosis in a coronary artery that corresponded to the region of ST-segment elevation on ECG. “Nonconclusive STEMI” was defined as cases with clinical presentation and ECG features suggestive of STEMI but with the absence of a culprit lesion on coronary angiography. Under the direction of the treating physician, additional investigations were conducted as appropriate to exclude life-threatening conditions (e.g., computed tomography, echocardiography, additional blood tests) before transfer to the referral hospital, or, for patients from our local catchment area, to identify a final diagnosis for causes of false-positive STEMI.
The Western Denmark Heart Registry collects baseline characteristics and patient- and procedure-specific information on all angiographies and coronary interventions performed in the region. From this database, we extracted all acute activations of our catheterization laboratory. We reviewed medical records for all these patients to determine the indication for the acute cardiac catheterization. In all cases, the data from the Western Denmark Heart Registry was manually supplemented with information from patient medical records and cross-checked by the authors.
Results
The flow chart of 917 acute angiograms performed during the study period is shown in Figure 1 . Of these, 117 angiograms were indicated by out-of-hospital cardiac arrest without reported preceding chest pain, and 185 angiograms were due to other indications, primarily other acute coronary symptoms that could not be stabilized by medical therapy. Of the 615 patients with suspected STEMI who underwent angiography, 483 (79%) patients had conclusive STEMI and 132 (21%) patients did not have conclusive STEMI.
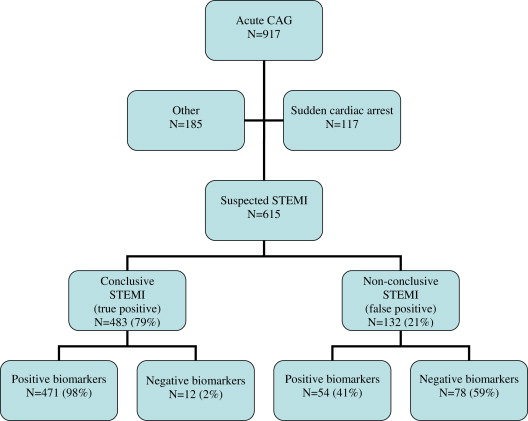
Baseline characteristics and outcomes between patients with and without conclusive STEMI are shown in Table 1 . A statistically higher proportion of patients with conclusive STEMI were men, and patients without conclusive STEMI were more likely to have diabetes, left bundle branch block, hypertension, or a history of coronary artery bypass surgery. Compared with the overall 79% with conclusive STEMI, patients with left bundle branch block or a history of coronary artery bypass surgery had positive predictive values of only 26% and 41%, respectively.
| Variable | Total (n = 615) | True Positive (n = 483) | False Positive (n = 132) | p Value |
|---|---|---|---|---|
| Age, mean ± SD (yrs) | 64 ± 14 | 64 ± 13 | 62 ± 16 | 0.19 |
| Male gender | 431 (70%) | 352 (73%) | 79 (60%) | 0.004 |
| Hypertension | 226 (39%) | 165 (36%) | 61 (48%) | 0.011 |
| Diabetes | 64 (11%) | 44 (10%) | 20 (16%) | 0.043 |
| Statin treatment | 160 (27%) | 122 (26%) | 38 (30%) | 0.37 |
| Smoker | 243 (44%) | 200 (46%) | 43 (37%) | 0.09 |
| Previous myocardial infarction | 70 (12%) | 51 (11%) | 19 (15%) | 0.22 |
| Previous percutaneous coronary intervention | 71 (12%) | 55 (12%) | 16 (12%) | 0.82 |
| Previous coronary artery bypass graft | 17 (3%) | 7 (2%) | 10 (8%) | 0.001 |
| Left bundle branch block | 43 (7%) | 11 (2%) | 32 (24%) | <0.001 |
| Positive biomarkers | 525 (86)% | 471 (98%) | 54 (41%) | <0.001 |
| Ejection fraction, mean ± SD | 47 ± 12% | 47 ± 11% | 50 ± 14% | 0.23 |
Of the 483 patients with conclusive STEMI, 471 (98 %) had positive biomarkers. Serial biomarkers were not available in 5 patients with conclusive STEMI as they were only measured in 1 blood sample taken immediately on arrival at the catheterization laboratory; 1 patient with cardiogenic shock died before blood samples for biomarkers were obtained; and 6 patients had negative serial biomarkers despite an assumed culprit lesion; 4 of these had an ECG with ST-segment elevations, and 2 had left bundle branch block.
The patients in the conclusive STEMI group consisted of 277 (57%) patients with 1-vessel disease, 114 (24%) patients with 2-vessel disease, and 92 (19%) patients with 3-vessel disease. The patients in the nonconclusive STEMI group consisted of 74 (56%) patients with normal coronary arteries, 16 (12%) patients with 1-vessel disease, 8 (6%) patients with 2-vessel disease, 13 (10%) patients with 3-vessel disease, and 21 (16%) patients with diffuse coronary artery disease without significant stenoses.
Of the 132 patients without conclusive STEMI, 54 (41%) had positive and 78 (59%) had negative cardiac biomarker results. The suspected etiologies are listed in Table 2 .



