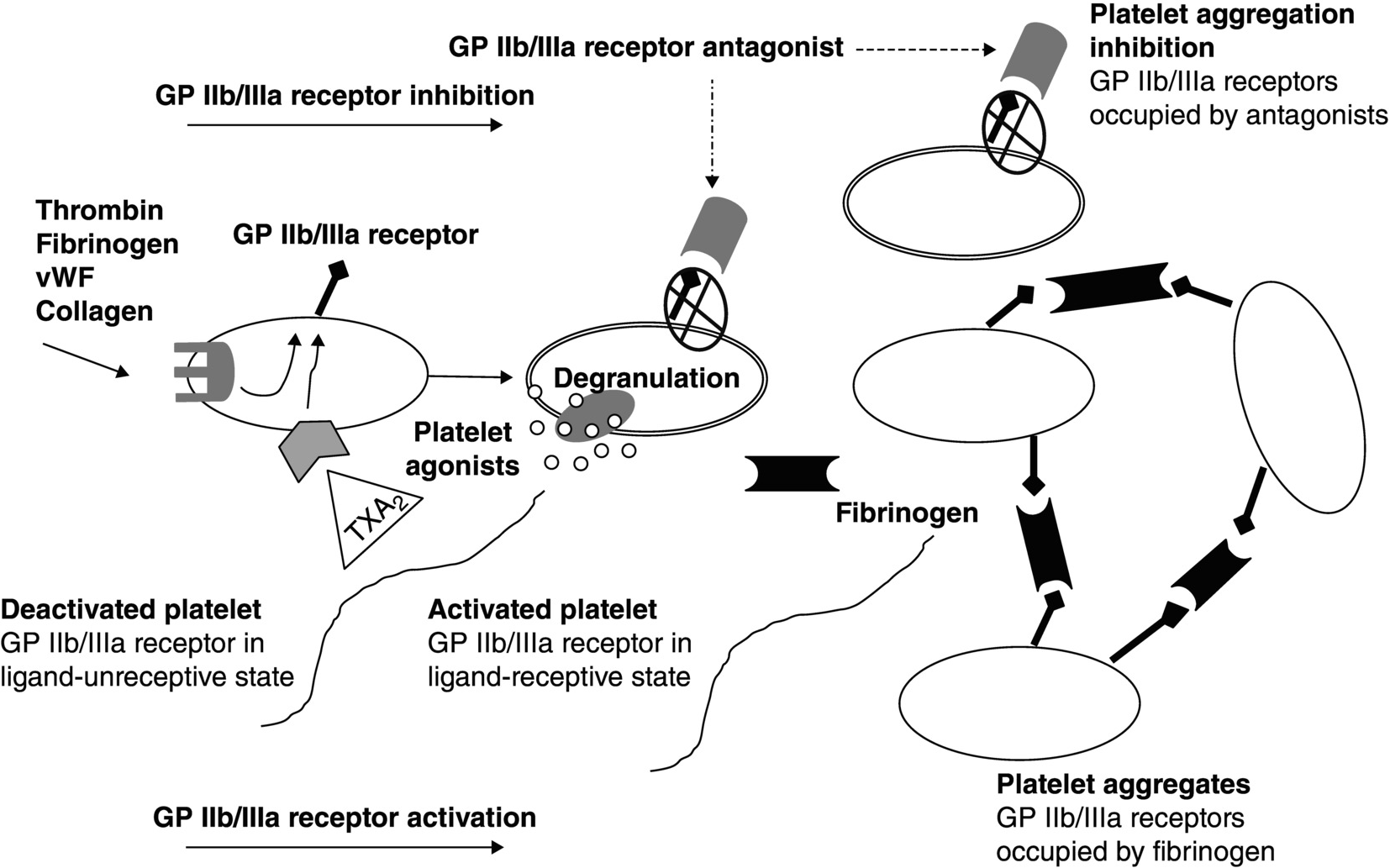
4 Eliano Pio Navarese1,2 and Jacek Kubica1,2 1 Ludwik Rydygier Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland 2 Systematic Investigation and Research on Interventions and Outcomes (SIRIO)-MEDICINE Research Network The platelet glycoprotein (GP) IIb/IIIa receptor has been identified as a target for control of the platelet response to vascular injury [1]. During the last decade, intensive efforts have been made to evaluate the role of the GPIIb/IIIa complex in platelet-mediated thrombus formation. GPIIb/IIIa is a calcium-dependent complex consisting of two components: α-subunit (GPIIb) and β-subunit (GPIIIa), noncovalently associated together. GPIIIa is a simple chain, while GPIIb is formed by two polypeptide chains connected by one or more disulfide bounds [2, 3]; both the α- and β-subunits are noncovalently bound to each other, and calcium is required to maintain the heterodimeric structure. The complex, also known as αIIbβ3 integrin, belongs to a group of cell-surface receptors initiating multiple cell–cell and cell-surface adhesions. Over 70% of the GPIIb/IIIa is placed on the plasma membrane of platelets, while the rest is stored between membranes of the surface and cytoplasmic α-granules, from which it is released after stimulation of the platelet [4]. Activated platelets play a pivotal role in thrombotic processes. There are three phases in the response of platelets to tissue injury. Phase 1 is adhesion, commonly triggered by plaque rupture, which stimulates platelets to adhere to the subendothelial matrix; during acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI), intimal injury can be generated causing damage to endothelium and leading to exposure of collagen and subendothelial molecules. Platelets adhere to exposed collagen, von Willebrand factor (vWF), and fibrinogen by specific cell receptors. Adherent platelets can then be activated by thrombin, collagen, thromboxane, serotonin, epinephrine, and adenosine diphosphate (ADP) – phase 2. Activated platelets degranulate and secrete various substances, including chemotaxins, clotting factors, vasoconstrictors, platelet activators (such as thromboxane A2 (TXA2), serotonin, ADP, and epinephrine), and mediators of inflammation and vascular repair. Platelet activation also includes changes in the platelet surface that promote coagulation and aggregation – phase 3. Regardless of the mode of platelet activation, GPIIb/IIIa serves as the receptor on platelets that binds plasma-borne adhesive proteins, such as fibrinogen and vWF, thus permitting platelet aggregation. GPIIb/IIIa receptor antagonists act by inhibiting this final common pathway of platelet aggregation. Platelet activation causes modifications in the shape of platelets and conformational changes in the GPIIb/IIIa receptor, transforming it from a ligand-unreceptive to a ligand-receptive state. The GPIIb/IIIa receptors on most platelet surfaces are presented as deactivated forms that require activation by thrombin, collagen, or TXA2; platelet aggregation begins when an aggregating agent interacts with its receptor(s) on the platelet surface. Ligand-receptive GPIIb/IIIa receptors bind fibrinogen molecules, which establish bridges between adjacent platelets and facilitate platelet aggregation (Figure 4.1). Although binding of fibrinogen to GPIIb/IIIa receptors is the principal mechanism for platelet aggregation, other adhesive GPs including vWF, fibronectin, and vitronectin also bind to these receptors. Fibrinogen and vWF are able to form bridges between stimulated platelets and therefore are responsible for generation of platelet aggregates; specifically, platelet aggregation involves cross-linking of activated GPIIb/IIIa receptors on two adjacent platelets by a single molecule of plasma fibrinogen [5]. Several efforts have been made to explain the molecular basis for binding of the soluble adhesive proteins of vWF and fibrinogen to platelets; the GPIIb/IIIa receptor has two peptide sequences, through which the agonists bind to it. One is the Arg–Gly–Asp (RGD); the other sequence is Lys–Gln–Ala–Gly–Asp–Val (KQAGDV). The RGD sequence is present in fibrinogen, vWF, and vitronectin. Unlike RGD, KQAGDV sequence is found only in fibrinogen and is probably the predominant site for the binding of fibrinogen to GPIIb/IIIa receptors. Figure 4.1 Role of GPIIb/IIIa receptor in platelet physiology and inhibition of platelet activation–aggregation by GPIIb/IIIa antagonists. GP, glycoprotein; vWF, von Willebrand factor. GPIIb/IIIa receptor expression and function are dynamic and responsive to the state of internal activation of platelets. “Inside-out” GPIIb/IIIa signaling has been described to trigger platelet activation and increase receptor expression and binding affinity [6]. First platelet–platelet contacts that initiate aggregation occur after fibrinogen and vWF binding to the GPIIb/IIIa receptor. These contacts are initially reversible, but at further stages, they lead to “outside-in” GPIIb/IIIa signaling that eventually stabilizes the platelet aggregates [7]. Indeed, it has been shown that the binding of the GPIIb/IIIa receptor initiates an outside-to-inside signal that prompts platelets to release the contents of their cytoplasmic granules which include adhesive molecules, growth factors, and procoagulant substances as well as to synthesize and release TXA2
Platelet Receptors and Drug Targets: GP IIb/IIIa
Introduction
Biology of the receptor
Glycoprotein IIb/IIIa receptor in platelet physiology
Platelet aggregation phase
“Inside-out” and “outside-in” GPIIb/IIIa signaling phenomenon
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


