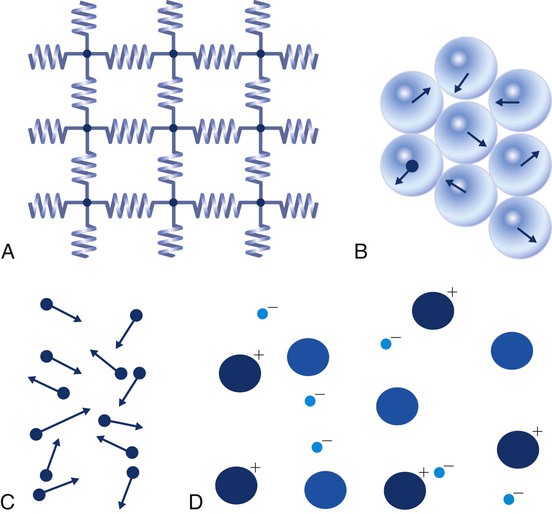
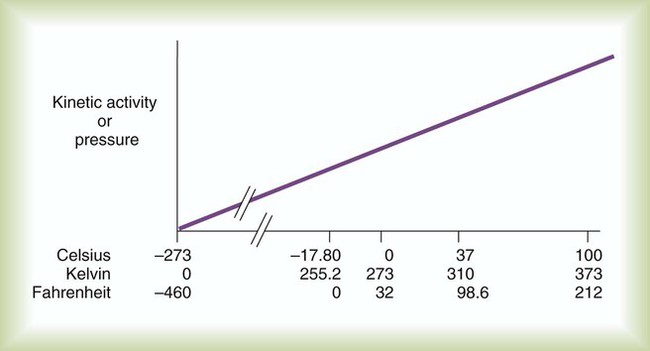
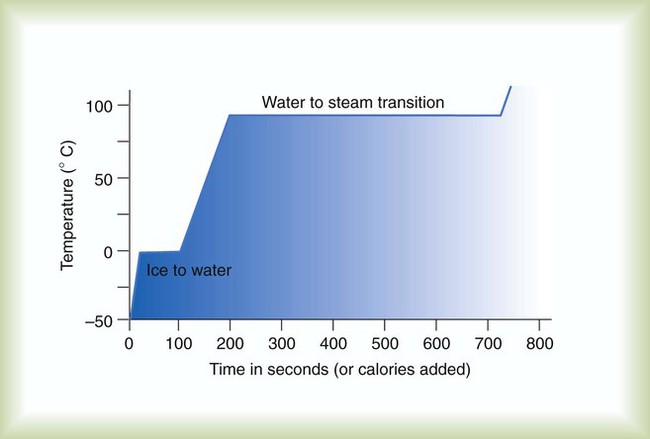
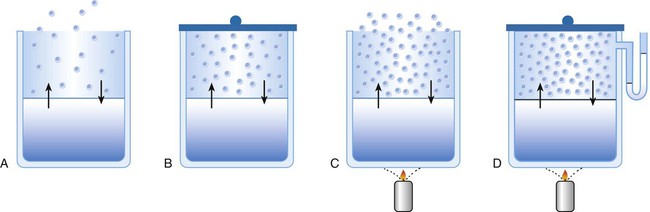
After reading this chapter you will be able to: There are three primary states of matter: solid, liquid, and gas. Figure 6-1, A-C depicts simplified models of these states of matter. Solids have a fixed volume and shape. The molecules that make up the solid have the shortest distance to travel until they collide with one another. This motion has been referred to as a “jiggle.” Solids have a high degree of internal order; their atoms or molecules are limited to back-and-forth motion about a central position, as if held together by springs (see Figure 6-1, A). Solids maintain their shape because their atoms are kept in place by strong mutual attractive forces, called van der Waals forces.1 Liquids have a fixed volume, but adapt to the shape of their container. If a liquid is not held within a container, the shape is determined by numerous internal and external forces. Liquid molecules exhibit mutual attraction. However, because these forces are much weaker in liquids than in solids, liquid molecules can move about freely (see Figure 6-1, B). This freedom of motion explains why liquids take the shape of their containers and are capable of flow. However, similar to solids, liquids are dense and cannot be compressed easily. In a gas, molecular attractive forces are very weak. Gas molecules, which lack restriction to their movement, exhibit rapid, random motion with frequent collisions (see Figure 6-1, C). Gases have no inherent boundaries and are easily compressed and expanded. Similar to liquids, gases can flow. For this reason, both liquids and gases are considered fluids. Gases have no fixed volume or shape. Both of these qualities depend on local conditions for the gas. Plasma has been referred to as a fourth state of matter. Plasma is a combination of neutral atoms, free electrons, and atomic nuclei. Plasmas can react to electromagnetic forces and flow freely similar to a liquid or a gas (see Figure 6-1, D). Although mentioned here for the sake of completeness, plasmas are not discussed further because at this time they are not known to be relevant to the practice of respiratory care. The atoms of all matter, at ordinary temperatures, are in constant motion.2 All matter has some kinetic energy. However, most internal energy in solids and liquids is potential energy. This potential energy is a result of the strong attractive forces between molecules. These intermolecular forces cause rigidity in solids and cohesiveness and viscosity in liquids. In contrast, because these attractive forces are so weak in gases, most internal energy in gases is kinetic energy. Heat transfer between objects is quantified by using a measure called thermal conductivity. Table 6-1 lists the thermal conductivities of selected substances in cgs (centimeter-gram-second) system units. As is evident, solids, in particular, metals, tend to have high thermal conductivity. This is why metals feel cold to the touch even when at room temperature. In this case, the high thermal conductivity of metal quickly draws heat away from the skin, creating a feeling of “cold.” In contrast, with fewer molecular collisions than in solids and liquids, gases exhibit low thermal conductivity. TABLE 6-1 Thermal Conductivities in (cal/sec)/(cm2 °C/cm) From Nave CR, Nave BC: Physics for the health sciences, ed 3, Philadelphia, 1985, WB Saunders. Heat transfer in both liquids and gases occurs mainly by convection. Convection involves the mixing of fluid molecules at different temperatures. Although air is a poor heat conductor (see Table 6-1), it can efficiently transfer heat by convection. To do so, the air is first warmed in one location and then circulated to carry the heat elsewhere; this is the principle behind forced-air heating in houses and convection heating in infant incubators. Fluid movements carrying heat energy are called convection currents. Three physical principles describe how energy is handled and transferred. These principles are known as the laws of thermodynamics.3,4 1. The law of conservation of energy states that energy cannot be created or destroyed. This law is usually stated as follows: The total amount of energy in a system is equal to the amount of heat put into the system minus the work. 2. The principle of thermodynamic equilibrium is that given time all systems achieve the lowest possible energy state (entropy). 3. The third law is a statistical law describing the impossibility of achieving absolute zero. As stated in the third law, at absolute zero all processes cease, and entropy is at a minimum value. Two interrelated terms are significant when discussing thermodynamics: entropy and enthalpy. Entropy is the amount of energy in a system that is unavailable for work. Entropy is the lowest amount of organization that a system can achieve (chaos). Enthalpy is the total measure of energy in the system. Enthalpy can be considered to be the order of a system. Temperature and kinetic energy are closely related.2 Temperature is a measurement of heat. Heat is the result of molecules colliding with one another. The temperature of a gas, with most of its internal energy spent keeping molecules in motion, is directly proportional to its kinetic energy. In contrast, the temperatures of solids and liquids represent only part of their total internal energy. Multiple scales can be used to measure temperature. The Fahrenheit and Celsius scales are based on a property of water. A third scale, the Kelvin scale, is based on molecular motion. Absolute zero provides a logical zero point on which to build a temperature scale. The SI (International System of Units) units for temperature are measured in kelvin (K) with a lower case “k,” with a zero point equal to absolute zero (0° K).5–7 Because the Kelvin scale has 100 degrees between the freezing and boiling points of water, it is a centigrade, or 100-step, temperature scale. The Kelvin scale has the unique quality of being based on the triple point definition for water (the temperature where all three phases of water exist). This temperature happens to be approximately 273° K (0.0° C).5–7 Conversely, to convert degrees Kelvin to Celsius, you simply subtract 273. For example: To convert degrees Fahrenheit to degrees Celsius, use the following formula: To convert degrees Celsius to degrees Fahrenheit, simply reverse this formula: Figure 6-2 shows the relationship between the kinetic activity of matter and temperature on all three common temperature scales. For ease of reference, five key points are defined: the zero point of each scale, the freezing point of water (0° C), body temperature (37° C), and the boiling point of water (100° C). The changeover from the solid to liquid state is called melting. The temperature at which this changeover occurs is the melting point.2 The range of melting points is considerable. For example, water (ice) has a melting point of 0° C, carbon has a melting point of greater than 3500° C, and helium has a melting point of less than −272° C. Figure 6-3 depicts the phase change caused by heating water. At the left origin of −50° C, water is solid ice. As the ice is heated, its temperature increases. At its melting point of 0° C, ice begins to change into liquid water. However, the full change to liquid water requires additional heat. This additional heat energy changes the state of water but does not immediately change its temperature. Clinically, Archimedes’ principle is used to measure the specific gravity of certain liquids. The term specific gravity refers to the ratio of the density of one fluid compared with the density of another reference substance, which is typically water. Figure 6-5 shows the use of a hydrometer to measure the specific gravity of urine. The specific gravity of gases also can be measured. In this case, oxygen or hydrogen is used as the standard instead of water. Gases also exert buoyant force, although much less than that provided by liquids. Buoyancy helps keep solid particles suspended in gases. These suspensions, called aerosols, play an important role in respiratory care. More detail on the characteristics and use of aerosols is provided in Chapter 35. Viscosity is most important when fluids move in discrete cylindrical layers, called streamlines. This pattern of motion is called laminar flow. As shown in Figure 6-6, frictional forces between the streamlines and the tube wall impede movement of the outer layers of a fluid. Each layer, moving toward the center of the tube, hinders the motion of the next inner layer less and less. Laminar flow consists of concentric layers of fluid flowing parallel to the tube wall at velocities that increase toward the center. The attractive force between like molecules is called cohesion. The attractive force between unlike molecules is called adhesion. These forces can be observed at work by placing a liquid in a small-diameter tube. As shown in Figure 6-7, the top of the liquid forms a curved surface, or meniscus. When the liquid is water, the meniscus is concave because the water molecules at the surface adhere to the glass more strongly than they cohere to each other (see Figure 6-7A). In contrast, a mercury meniscus is convex (see Figure 6-7B). In this case, the cohesive forces pulling the mercury atoms together exceed the adhesive forces trying to attract the mercury to the glass. Surface tension is a force exerted by like molecules at the surface of a liquid. A small drop of fluid provides a good illustration of this force. As shown in Figure 6-8, cohesive forces affect molecules inside the drop equally from all directions. However, only inward forces affect molecules on the surface. This imbalance in forces causes the surface film to contract into the smallest possible surface area, usually a sphere or curve (meniscus). This phenomenon explains why liquid droplets and bubbles retain a spherical shape. Surface tension is quantified by measurement of the force needed to produce a “tear” in a fluid surface layer. Table 6-2 lists the surface tensions of selected liquids in dynes/cm (cgs). For a given liquid, surface tension varies inversely with temperature: The higher the temperature, the lower is the surface tension. TABLE 6-2 P is the pressure in the bubble, ST is the surface tension, and r is the bubble radius. Figure 6-9 shows this relationship for two bubbles of different sizes, each with the same surface tension. Because the alveoli of the lungs resemble clumps of bubbles, it follows that surface tension plays a key role in the mechanics of ventilation (see Chapter 10). Abnormalities in alveolar surface tension occur in certain clinical conditions, such as acute respiratory distress syndrome. These abnormalities may result in collapse of alveoli secondary to high surface tension. Capillary action is a phenomenon in which a liquid in a small tube moves upward, against gravity. Capillary action involves both adhesive and surface tension forces. As shown in Figure 6-10, A, the adhesion of water molecules to the walls of a thin tube causes an upward force on the edges of the liquid and produces a concave meniscus. Because surface tension acts to maintain the smallest possible liquid-gas interface, instead of just the edges of the liquid moving up, the whole surface is pulled upward. How strong this force is depends on the amount of liquid that contacts the tube’s surface. Because a small capillary tube creates a more concave meniscus and a greater area of contact, liquid rises higher in tubes with smaller cross-sectional areas (see Figure 6-10, B). Only after ice completely melts does additional heat increase the temperature of the newly formed liquid (see Figure 6-3). As the water temperature reaches 100° C, a new change of state begins—from liquid to vapor. This change of state is called vaporization. There are two different forms of vaporization: boiling and evaporation. Melting weakens attractive forces between molecules, whereas vaporization eliminates them. Elimination of these forces converts essentially all of the internal energy of a substance into kinetic energy. For this reason, vaporization requires substantially more energy than melting. As shown in Figure 6-3, almost seven times more energy is needed to convert water to steam (540 cal/g) than is needed to melt ice. Boiling is only one type of vaporization. A liquid also can change into a gas at temperatures lower than its boiling point through a process called evaporation. Water is a good example (Figure 6-11). When at a temperature lower than its boiling point, water enters the atmosphere via evaporation. The liquid molecules are in constant motion, as in the gas phase. Although this kinetic energy is less intense than in the gaseous state, it allows some molecules near the surface to escape into the surrounding air as water vapor (see Figure 6-11, A). If the container is covered, water vapor molecules continue to enter the air until it can hold no more water (see Figure 6-11, B). At this point, the air over the water is saturated with water vapor. However, vaporization does not stop when saturation occurs. Instead, for every molecule escaping into the air, another returns to the water reservoir. These conditions are referred to as a state of equilibrium. Second, if water is heated, its kinetic energy is increased, and more molecules are helped to escape from its surface (see Figure 6-11, C). Last, if the container of heated water is covered, the air again becomes saturated (see Figure 6-11, D). However, the heated saturated air, compared with the unheated air (see Figure 6-11, B), now contains more vapor molecules and exerts a higher vapor pressure (as shown by the manometer in Figure 6-11, D). The temperature of a gas affects both its capacity to hold molecular water and the water vapor pressure. The relationship between water vapor pressure and temperature is shown graphically in Figure 6-12. The left vertical axis plots water vapor pressure in both mm Hg and kPa (kilopascal). The horizontal axis plots temperatures between 0° C and 70° C. This graph shows that the greater the temperature, the greater the saturated water vapor pressure (bold red dots). Table 6-3 lists actual water vapor pressures in saturated air in the clinical range of temperatures (20° C to 37° C). TABLE 6-3 Water Vapor Pressures and Contents at Selected Temperatures Absolute humidity (AH) can be measured by weighing the water vapor extracted from air using a drying agent. Alternatively, absolute humidity can be computed with meteorologic data according to the techniques of the U.S. Weather Bureau. The common unit of measure for absolute humidity is milligrams of water vapor per liter of gas (mg/L). Absolute humidity values for saturated air at various temperatures are plotted against the right vertical axis of Figure 6-12, using hash marks. The middle column of Table 6-3 lists these absolute humidity values for saturated air between 20° C and 37° C. A gas does not need to be fully saturated with water vapor. If a gas is only half saturated with water vapor, its water vapor pressure and absolute humidity are only half that in the fully saturated state. Air that is fully saturated with water vapor at 37° C and 760 mm Hg has a water vapor pressure of 47 mm Hg and an absolute humidity of 43.8 mg/L (see Table 6-3). However, if the same volume of air were only 50% saturated with water vapor, its water vapor pressure would be 0.50 × 47 mm Hg, or 23.5 mm Hg, and its absolute humidity would be 0.50 × 43.8 mg/L, or 21.9 mg/L.
Physical Principles of Respiratory Care
 Describe the properties that characterize the three states of matter.
Describe the properties that characterize the three states of matter.
 Describe how heat transfer occurs among substances.
Describe how heat transfer occurs among substances.
 Identify the three common temperature scales and explain how to use them.
Identify the three common temperature scales and explain how to use them.
 Describe how substances undergo change of state.
Describe how substances undergo change of state.
 Identify the factors that influence the vaporization of water.
Identify the factors that influence the vaporization of water.
 Describe how water vapor capacity, absolute humidity, and relative humidity are related.
Describe how water vapor capacity, absolute humidity, and relative humidity are related.
 Describe how to predict gas behavior under changing conditions, including at extremes of temperature and pressure.
Describe how to predict gas behavior under changing conditions, including at extremes of temperature and pressure.
States of Matter
Internal Energy of Matter
Heat Transfer
Conduction
Material
Thermal Conductivity (k)
Silver
1.01
Copper
0.99
Aluminum
0.50
Iron
0.163
Lead
0.083
Ice
0.005
Glass
0.0025
Concrete
0.002
Water at 20° C
0.0014
Asbestos
0.0004
Hydrogen at 0° C
0.0004
Helium at 0° C
0.0003
Snow (dry)
0.00026
Fiberglass
0.00015
Cork board
0.00011
Wool felt
0.0001
Air at 0° C
0.000057
Convection
Laws of Thermodynamics
Internal Energy and Temperature
Temperature Scales
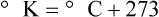
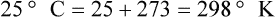
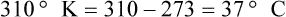
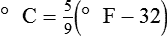
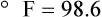
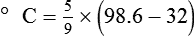
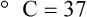
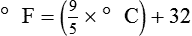
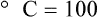
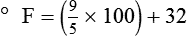
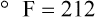
Change of State
Liquid-Solid Phase Changes (Melting and Freezing)
Melting
Properties of Liquids
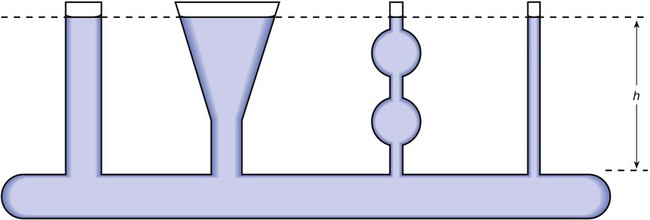
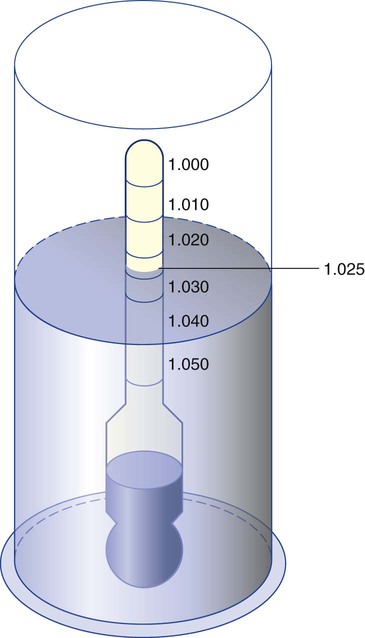
Buoyancy (Archimedes’ Principle)
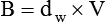
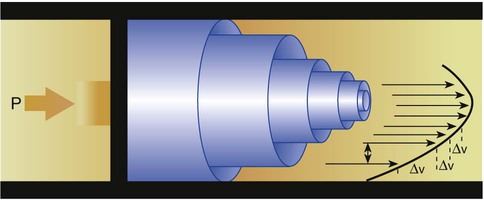
Viscosity
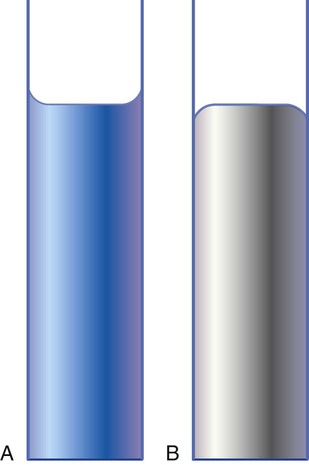
Cohesion and Adhesion
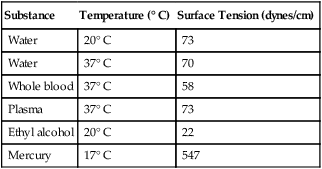
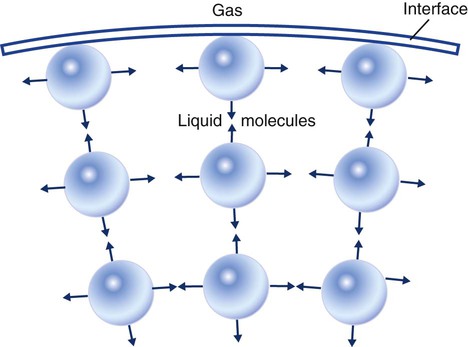
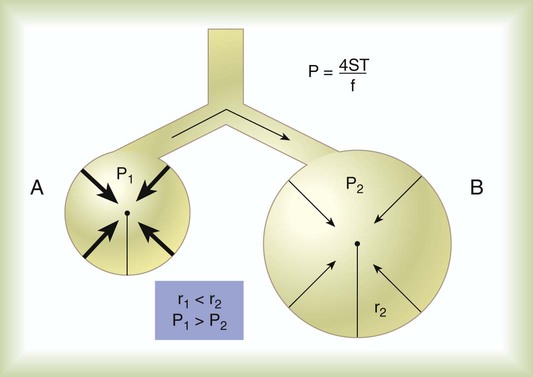
Surface Tension
Substance
Temperature (° C)
Surface Tension (dynes/cm)
Water
20° C
73
Water
37° C
70
Whole blood
37° C
58
Plasma
37° C
73
Ethyl alcohol
20° C
22
Mercury
17° C
547
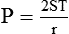
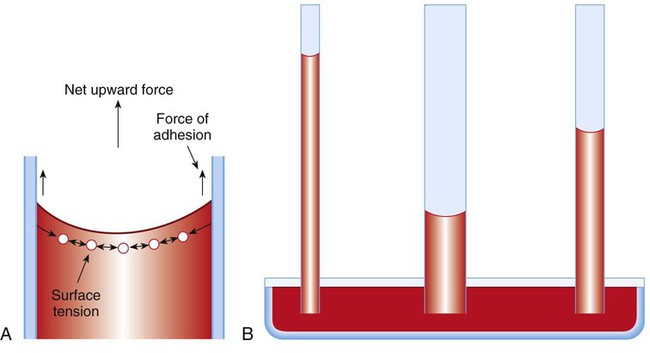
Capillary Action
Liquid-Vapor Phase Changes
Boiling
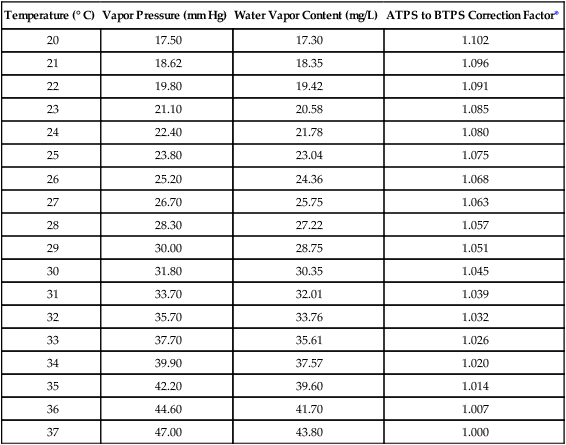
Evaporation, Vapor Pressure, and Humidity
Influence of Temperature
Temperature (° C)
Vapor Pressure (mm Hg)
Water Vapor Content (mg/L)
ATPS to BTPS Correction Factor*
20
17.50
17.30
1.102
21
18.62
18.35
1.096
22
19.80
19.42
1.091
23
21.10
20.58
1.085
24
22.40
21.78
1.080
25
23.80
23.04
1.075
26
25.20
24.36
1.068
27
26.70
25.75
1.063
28
28.30
27.22
1.057
29
30.00
28.75
1.051
30
31.80
30.35
1.045
31
33.70
32.01
1.039
32
35.70
33.76
1.032
33
37.70
35.61
1.026
34
39.90
37.57
1.020
35
42.20
39.60
1.014
36
44.60
41.70
1.007
37
47.00
43.80
1.000
Humidity
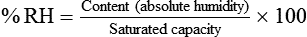
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




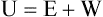
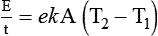
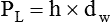
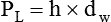
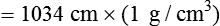
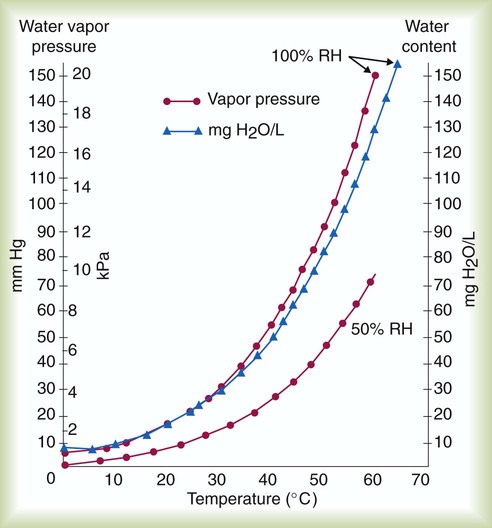
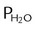 ) and absolute humidity (mg H2O/L) curves for gas that is fully saturated (relative humidity [RH] = 100%) and gas that is half saturated (RH = 50%).
) and absolute humidity (mg H2O/L) curves for gas that is fully saturated (relative humidity [RH] = 100%) and gas that is half saturated (RH = 50%).