Chapter 23 Photodynamic Therapy for Palliation of Infiltrative Endoluminal Obstruction at Left Secondary Carina and Left Lower Lobe Bronchus
Case Description
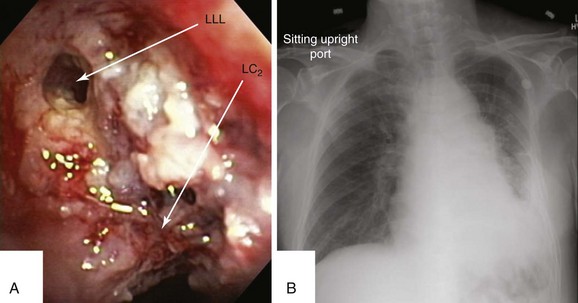
This patient was a 75-year-old man with stage IIIA squamous cell carcinoma who presented with worsening dyspnea, hemoptysis, and wheezing. A bronchoscopy performed by his pulmonologist showed near complete left lower lobe bronchial obstruction. He had undergone three cycles of chemotherapy, which were poorly tolerated. He refused further chemotherapy but agreed to receive external beam radiation therapy (EBRT), which unfortunately was complicated by radiation pneumonitis. Repeat bronchoscopy several weeks later showed recurrence of the tumor, prompting high-dose brachytherapy (dose and fractions unknown) at an outside facility. Follow-up bronchoscopy showed diffuse infiltrative tumor at the left secondary carina (LC2) nearly completely occluding the left lower lobe (LLL) bronchus. The segments in the left upper lobe (LUL) were infiltrated but patent, and the LLL bronchus was circumferentially narrowed by inflamed, necrotic, and friable mucosa, which was likely responsible for his hemoptysis and wheezing (Figure 23-1). His limited pulmonary function test (PFT) performed several months before showed FVC (forced vital capacity) of 2.26 L (69% of predicted), FEV1 (forced expiratory volume in one second) of 1.73 L (74% of predicted), and DLCO (diffusing capacity of the lungs for carbon monoxide) 68% of predicted. He was on no medications. On examination, the patient was cachectic, weighed 118 lbs, and had localized wheezes on the left side. After discussing various treatment alternatives, the patient decided to proceed with photodynamic therapy (PDT) to attempt restoration of LLL bronchial patency (see Figure 23-1).
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient had lobar obstruction due to endobronchial involvement from squamous cell carcinoma. Central airway obstruction from locally advanced lung cancer is seen in approximately 30% of patients previously treated with radiotherapy or chemoradiotherapy. This patient’s prior systemic chemotherapy and external beam radiation therapy (EBRT) did not preclude the use of photodynamic therapy (PDT) because PDT causes no direct injury to lung parenchyma. In one series, 85% of patients treated with PDT had received prior chemoradiotherapy.1 This patient’s previous brachytherapy did not preclude PDT either, and evidence indicates that the two modalities can be safely administered together or sequentially.2,3
Although bronchoscopy is essential in planning PDT, the response to PDT does not seem to correlate with the degree of airway obstruction or with tumor pathology. Preoperatively, the patient’s chest radiograph showed left lower lobe consolidation (see Figure 23-1); however, during initial evaluation of these patients, a computed tomography (CT) of the chest is probably preferable because CT may provide information regarding the extent of peribronchial involvement and airway distortion, which can be underestimated by bronchoscopy alone. Ventilation-perfusion studies may show absence or reduction of regional perfusion out of proportion to ventilation in the involved lung zone—a finding usually associated with extensive peribronchial involvement and poor outcome.4 Identification of significant peribronchial disease on CT has therapeutic implications because this is not treated using debulking techniques, and even if the airways are reopened, gas exchange may not improve if a reduction in local perfusion is due to tumor infiltration of the pulmonary vasculature.
Comorbidities
When considering PDT, physicians should be aware that patients with hepatic or renal impairment may need greater preventive measures for photosensitivity* because the commonly used photosensitizer, photofrin, is retained in the skin and in the reticuloendothelial system as well. In addition, interaction between photofrin† and other drugs is possible, and concomitant use of other photosensitizing agents such as tetracyclines, sulfonamides, phenothiazines, sulfonylurea hypoglycemic agents, thiazide diuretics, griseofulvin, and fluoroquinolones can increase the risk of photosensitivity reactions. Our patient had no evidence of hepatic or renal dysfunction and was on no medications.
Patient Preferences and Expectations
During the interview process, the patient wondered whether another treatment would be necessary, and if this could be safely repeated. We explained that during the cleanup bronchoscopy, performed a day or two after PDT, we could use the laser light to re-illuminate the lesion if persistent obstructing disease was present. In addition, two administrations of photofrin‡ within 30 to 45 days apart is possible if subsequent follow-up bronchoscopies continue to reveal symptomatic bronchial obstruction.
Procedural Strategies
Indications
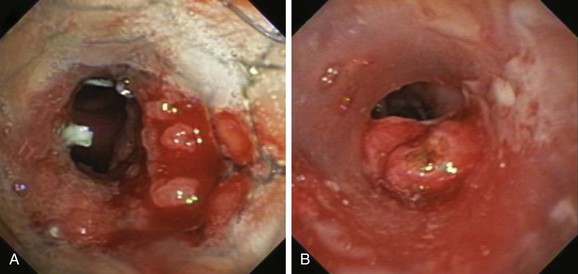
Described indications for PDT in the treatment of central airway obstruction include palliation of advanced malignant airway obstruction (especially chemoradiation-resistant lesions), tumor ingrowth through stents (Figure 23-2), and, rarely, benign disorders such as granulation tissue growth or papillomas.5,6 Accepted selection criteria for patients with locally advanced lung cancer consist of the following: (1) patients have inoperable or unresectable tumor with existing or impending related symptoms; (2) patients still have good performance status (Karnofsky Performance Status [KPS] index >50% or World Health Organization [WHO] scale ≤3); and (3) patients have no extrathoracic metastasis.7
PDT should be applied only to patients with intraluminal airway obstruction. Among these, the best response involves patients with mucosal involvement. Results from studies show that the mean duration of complete response was 22 weeks if more than 50% of the obstruction was due to mucosal disease, compared with only 7 weeks if the tumor was submucosal or extraluminal.4 In addition to the type of obstruction, the morphology (shape) of the treatment area leads to variations in response rates. In this regard, debulking and stent insertion add uniformity to the field of treatment by providing a more consistent shape and diameter of the obstructed airway. Although this could amplify PDT effect, airway stents may attenuate it by reducing light penetration.
Expected Results
In a review of 12 articles that describe a total of more than 600 patients with advanced lung cancer,8 bronchoscopic PDT was considered safe and effective for malignant endobronchial obstruction, providing symptomatic relief in all patients and a survival benefit for those without metastatic disease and with good performance status. Palliation of symptoms occurs in 74% to 100% of patients.5 Regardless of the pathology of the malignant obstruction (primary lung cancer, metastases), several factors influence the response to PDT. These include different optical properties of tissues that will influence the absorption of light and the depth of penetration (pigmentation, bleeding, and necrosis), extent of the lesion (superficial vs. “bulky” obstruction), and location of the lesion (large or small airways). Effects are less predictable with tumors located at acute angles, such as those noted in our case.
The basic principle of PDT is that the injected chemical (i.e., photofrin) accumulates more in rapidly growing tissues than in normal tissues. These molecules absorb light and produce cytotoxic oxygen species upon illumination with light of appropriate wavelength (600 to 800 nm).* This photochemical reaction leads to formation of reactive oxygen species, direct cell injury, and induction of apoptosis and vascular effects such as congestion, red cell extravasation within the tumor, and ischemic necrosis through neovascular shutdown mediated in part by thromboxane A2 release.9,10 The variability in clinical response noted in studies occurs when the interactions are inconsistent.
Results from randomized controlled studies* of patients with advanced-stage disease presented in abstract form show no survival advantage for PDT compared with neodymium-doped yttrium aluminum garnet (Nd:YAG) laser therapy, or PDT combined with radiotherapy compared with radiotherapy alone, but an advantage for PDT with radiotherapy was noted with respect to symptom control.11,12 One study randomized 211 patients to PDT or Nd:YAG laser therapy and found that the response was significantly greater 1 month after treatment with PDT than after treatment with Nd:YAG laser (55% vs. 30%).11 In another trial, the bronchial lumen was completely opened with no evidence of gross tumor in 14 of 20 patients receiving PDT plus radiotherapy compared with 2 of 21 patients receiving radiotherapy alone.12 Three of 20 patients experienced massive and fatal hemoptysis at 67, 187, and 567 days after treatment with PDT and radiotherapy,12 potentially suggesting that the addition of radiation after PDT may increase the risk for bronchoarterial fistula formation.
Noncontrolled studies have reported post-PDT reductions in dyspnea, hemoptysis, cough, or bronchial obstruction and atelectasis.13–16 One of these studies included 68 men and 32 women (mean age, 62.5 years) with advanced inoperable bronchogenic carcinoma and endobronchial obstruction; 43 patients had a functional WHO class of 2 or less, and 54 had a WHO class of greater than 2. Patients were followed and were re-treated on an as-needed basis every 6 to 8 weeks for 1 year, and then every 3 to 6 months until death; average PDT treatments per person numbered 1.47. Mean and median survival times for WHO of 2 or less were 17.8 and 14 months, respectively; for WHO of greater than 2, they were 6.9 and 4 months, respectively, potentially suggesting that PDT treatments should be offered to patients who are still functional with a reasonable performance status.16 When compared with Nd:YAG laser, PDT may offer a more durable response, and more patients may be symptom-free at 1 month in the PDT group.5
At least one study has shown that PDT may be better than Nd:YAG laser for symptom palliation in patients with central airway obstruction caused by non–small cell lung carcinoma (NSCLC).17 However, the two groups were unbalanced in terms of staging or severity of illness. This study included 31 patients (mean age, 65 years; KPS >40), and 57% of the PDT group had advanced NSCLC (IIIA, IV) versus 88% in the Nd:YAG laser group. Given these caveats, the study showed no difference between groups at 1 week in terms of symptoms or degree of obstruction, but the median time to treatment failure was 50 days for PDT versus 38 days for the Nd:YAG group (P = .03). Overall survival in this study favored the PDT group (265 vs. 95 days; P = .007), but again, this might be explained by the more advanced stage of disease in the Nd:YAG group.17
Expected complications after PDT include photosensitivity* (≈21%), which can be prolonged. Patients must avoid sun exposure for approximately 4 to 6 weeks after injection. Within days after treatment, however, airway obstruction may result from necrotic tissue, so a cleanup bronchoscopy† within 24 to 72 hours post intervention may be necessary when large obstructing lesions are treated. Other potential complications include dyspnea 30%, hemoptysis 16%, fever 16%, coughing 15%, pneumonia 12%, and bronchitis 10%.
Team Experience
Decisions about PDT probably should result from deliberations of a multidisciplinary lung cancer conference or clinic, including surgery, radiation oncology, pulmonary, and oncology subspecialties. An institution that offers PDT should have a readily available bronchoscopy team because PDT may require salvage bronchoscopies performed within 48 hours post illumination for tumor slough removal.‡ The bronchoscopy team should also be readily available during the treatment period because the risk of fatal hemoptysis is increased, especially in patients who have undergone multiple endobronchial procedures, previous radiation therapy, or metal stent placement.
The most common adverse effect related to PDT is skin photosensitivity, with an incidence between 5% and 28%. Lesser rates were reported from centers that had a dedicated member of the team assigned to provide counseling and information to patients.8 Great value is derived from having a dedicated PDT nurse to offer patient education and to explain the importance of adhering to protection from sunlight strategies.*
Therapeutic Alternatives
For patients with malignant obstruction, apart from traditional surgical resection, radiotherapy, and chemotherapy, several bronchoscopic options are available. Nd:YAG laser, brachytherapy, and cryotherapy have been used alone or in combination for symptom palliation. Rigid bronchoscopy with laser photocoagulation and coring out of the tumor is especially useful for tracheal or mainstem bronchial disease and when hemoptysis is present. In our patient, the lobar obstruction caused by mucosal disease was not appropriate for laser treatment because of a substantial infiltrative rather than endoluminal exophytic component. In addition, although stent insertion is an alternative in patients with malignant obstruction, our patient had a distal lobar location of disease (LLL) that was not ideal for stent placement because the LLL bronchus is both short and narrow. Cryotherapy usually preserves airway cartilage and was an alternative for this patient18; however, it was not ideal because of the absence of substantial exophytic disease. Further high-dose brachytherapy was not warranted because it had already been attempted without success.
Cost-Effectiveness
Since 1988, PDT has been approved by the U.S. Food and Drug Administration for palliative treatment of central airway lesions due to NSCLC.19 In 2004, the National Institute for Clinical Excellence stated, “Current evidence on safety and efficacy of PDT for advanced bronchial carcinoma is adequate to support its use to treat patients with inoperable NSCLC.”20
Techniques and Results
Anesthesia and Perioperative Care
Studies report flexible bronchoscopic PDT with patients under moderate sedation. Some studies have used general anesthesia and rigid bronchoscopy or endotracheal tube for ventilation, through which operators place the flexible instrument for tumor location and illumination. Use of general anesthetic and the rigid bronchoscope in patients with bronchial obstruction undergoing PDT allows good access for debridement, thereby achieving a thorough cleaning of the bronchial tree and ample room to maneuver in case of complications.8 In the opinion of many PDT experts, combined rigid and flexible instruments under general anesthesia are best for patients with obstructive tumors of the trachea and mainstem bronchi, but for other cases, the method depends on the experience of the operator and a patient’s comorbidities and personal choice.7 We decided to perform this particular procedure on our patient while under general anesthesia to allow better control of the diffuser in the distal left main and lower lobe bronchi and to have a secure airway in case the patient’s hemoptysis worsened during the procedure.
Technique and Instrumentation
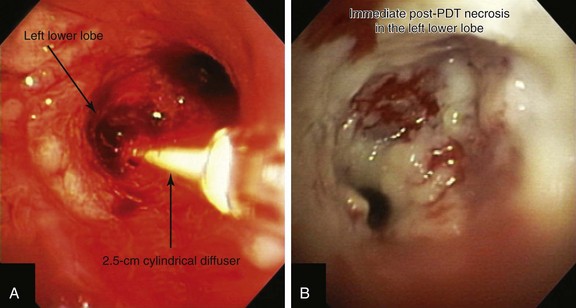
Light is delivered to the tumor by cylindrical fiberoptic diffusers passed through the working channel of the bronchoscope (Figure 23-3). The fiber may be positioned alongside the tumor when it is relatively flat, or it can be implanted within the tumor when it is bulky and exophytic. Cylindrical diffusers are commonly used for central airway obstruction and are available in several lengths; the choice of diffuser tip length* depends on the length of the tumor to be treated. Diffuser length should be properly sized to avoid exposure of nonmalignant tissue to light and to prevent overlapping of previously treated malignant tissue. Therefore, before light administration, the length of the tumor is assessed with bronchoscopy, and a suitable light fiber length is selected to transilluminate the tumor. Within lung and tumor tissue, 630 nm light will penetrate to a depth of 5 to 10 mm from the point of origin, depending on the power density and the length of the fiber.21 Argon-pumped dye laser and diode-based systems have been used as a light source. We used a compact diode-based laser (Diomed Ltd., Andover, Mass) that was available at our institution. This is a nonthermal laser, so no risks of airway fire are present. Photoactivation of photofrin is controlled by the total light dose delivered. In the treatment of endobronchial cancer, a light dose of 200 J/cm of diffuser length should be delivered. The total power output at the fiber tip is set to deliver the appropriate light dose using exposure times of 8 minutes and 20 seconds.21
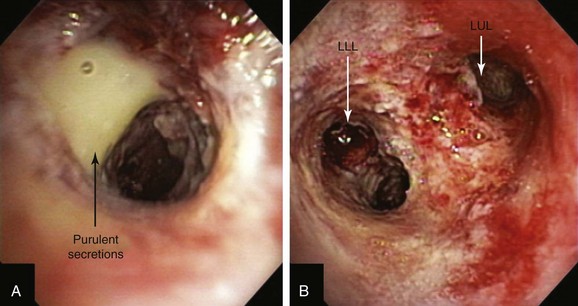
A step-by-step approach to bronchoscopic PDT proceeds as follows: First, the treatment plan is developed by performing diagnostic flexible bronchoscopy, during which time the length of the tumor is assessed and the diffuser length is selected (see Figure 23-1). Then, in the presensitization phase, photofrin is injected; after a latent period of 2 to 3 days (which permits optimal differential concentration between tumor and healthy surrounding tissue), some investigators measure drug levels by point spectroscopy; others proceed straight to the illumination (irradiative) phase* using laser light of appropriate wavelength (630 nm) (see Figure 23-3). Light doses of 200 J/cm of diffuser length are used in endobronchial cancer for both palliation of obstructing cancer and treatment of superficial lesions.22 One to three days later, debridement is usually necessary when obstructing lesions are treated (Figure 23-4); before a second laser light treatment is provided, any residual tumor should be debrided, which may cause bleeding if performed vigorously. In fact, debridement of necrotic tissue should be discontinued if the volume of bleeding increases; this may indicate that debridement has gone beyond the zone of the PDT effect. Re-illumination can then be performed if residual obstruction remains, usually within 96 to 120 hours after the initial injection, but no further injection of photofrin should be given for such re-treatment with laser light. In fact, most patients receive two treatments during their hospitalization, and many return several weeks later for repeated treatments.23
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree