Disease
B max alternation
Heart failure
→ ↓
Myocardial ischemia
→ ↑↓
Hypertension
→ ↑↓
Diabetes
↓
Cardiotoxicity
↓
The levels of β2-adrenoceptor and β2-adrenoceptor mRNA remain unaffected but it is believed that these receptors become uncoupled (Brodde 1991). Patients with severe left ventricular dysfunction showed fewer β-adrenergic receptors in lymphocytes, as measured in radioligand binding assays (Colucci et al. 1981). However, although changes in lymphocyte β2-adrenoceptors are significantly correlated with changes in cardiac β2-adrenoceptors, they are not related to changes in cardiac β1-adrenoceptors, which predominate in all parts of the human heart. Furthermore, circulating lymphocytes are not exposed to the local environment of neuronally released catecholamines in the myocardial interstitium. The use of lymphocyte β2-adrenoceptors as a tool for predicting the status of cardiac β-adrenoceptors is therefore quite limited (Brodde et al. 1989), and thus cardiac tissue will be needed to evaluate cardiac β-adrenoceptor function. Abnormal sympathetic nervous system and β-adrenoceptor signaling is also associated with diabetes. Thackeray and colleagues used [3H]-CGP-12177 to examine altered β-adrenoceptor expression in diabetic rat hearts (Thackeray et al. 2011). Reduced cardiac [3H]-CGP-12177 binding in the presence of sustained hyperglycemia corresponded to a decrease in relative β-adrenoceptor expression. Their study indirectly supports the use of [11C]-CGP-12177 for assessment of cardiac dysfunction in diabetes, by evaluating the cardiac β-adrenoceptor density.
11.4 Non-invasive Imaging of Cardiac β-Adrenergic Receptors
11.4.1 PET Imaging and Density Measurement of Cardiac β-Adrenergic Receptors
Several postsynaptic receptor ligands have been labeled and proposed as PET tracers for cardiac quantification and imaging (Elsinga et al. 1998; Law et al. 2010; Tseng et al. 2001). However, the clinical use of receptor-targeted tracers has been limited to a few studies and still faces significant challenge. High specific binding, high affinity, and hydrophilicity, which avoids binding to internalized inactive receptors, lack of pharmacologic effects, and, finally, a simple and reliable tracer synthesis, are requirements that must be met for a widespread application of receptor ligands for cardiac PET. [11C]-CGP-12177, a hydrophilic nonselective β-adrenoreceptor antagonist, is still the most widely used tracer for adrenergic receptor imaging (Caldwell et al. 2008; Elsinga et al. 1998; Link et al. 2003; Naya et al. 2009) Synthesis of this tracer is not simple and requires [11C]-phosgene as a precursor, which has prevented a broader clinical application until now. CGP-12177 has high receptor affinity and fast plasma clearance, suggesting feasibility for clinical imaging. A graphical method, which adjusts for kinetics related to metabolites, has been established for quantification in humans (Delforge et al. 2002). This approach requires a dual-injection protocol with tracer doses of high and low specific activity (Fig. 11.1). β-Adrenergic receptor density (B max) measured by [11C]-CGP-12177 PET correlated well with in vitro measurements of myocardial samples in both healthy volunteers and patients with congestive cardiomyopathy (Delforge et al. 2002). [11C]-CGP-12388 is a non-subtype-selective β-adrenergic receptor antagonist and an isopropyl analog of CGP-12177. CGP-12388 can be labeled easier than CGP-12177 via a one-step procedure using 2-[11C]-acetone (Elsinga et al. 1994). It is equally hydrophilic compared to [11C]-CGP-12177 and the biodistribution and retention of CGP-12388 is reported to be similar to CGP-12177 (Doze et al. 2002). Both CGP ligands have been applied in the biologically active S-enantiomer and can be blocked by pindolol (Fig. 11.1).
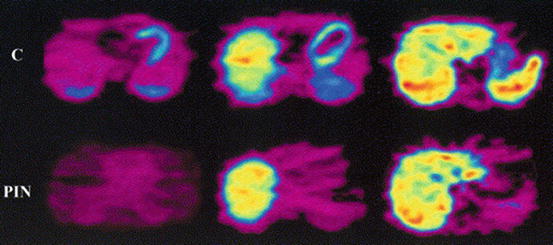

Fig. 11.1
PET images of a human volunteer acquired with [11C]-CGP-12388. Transaxial cross sections in the time frame 14–60 min postinjection are displayed. The upper row is the control study; the bottom row is the pindolol-blocked study (Elsinga et al. 2001)
[18F]-fluorocarazolol and [11C]-carazolol are non-subtype-selective, lipophilic radioligands with high affinity for β1– and β2-adrenoceptors. The use of fluorine-18 instead of carbon-11 has the advantages of higher specific activity and a longer half-life, which enables prolonged PET studies.
[11C]-carazolol has been evaluated by Berridge and coworkers in mice and pigs (Berridge et al. 1994). The pig heart was clearly visualized. Specific binding to β-adrenoceptors was demonstrated by injection of the bioactive [11C]-isomer (specific and nonspecific binding), followed by a second injection of the (R)-isomer (only nonspecific binding). [18F]-fluorocarazolol has been evaluated in several animal models and in humans. Specific binding to β-adrenoceptors of [18F]-fluorocarazolol was demonstrated: (1) by injection of the (S)-isomer and subsequent injection of the (R)-isomer, (2) by blocking experiments with various β-adrenoceptor agonists and antagonists (van Waarde et al. 1995), and (3) by saturation experiments (Doze et al. 1998). The in vivo binding of fluorocarazolol was found to be stereospecific (activity residing in the (S)-isomer). It could be blocked by drugs that bind to β1– and β2-adrenoceptors, and specific binding was in good agreement with β-adrenoceptor densities determined by in vitro assays. Metabolite analyses of [18F]-fluorocarazolol showed a rapid (<5 min) appearance of polar metabolites in plasma, while at 60 min postinjection, 92 and 82 % of the total radioactivity in lung and heart remained native [18F]-fluorocarazolol (van Waarde et al. 1995). In PET images of male Wistar rats, the lungs were clearly visible and pulmonary uptake of radioactivity was strongly decreased (>90 %) after pretreatment of the animals with propranolol. The heart could not be visualized. However, PET scans after i.v. injection of [18F]-fluorocarazolol in human volunteers clearly showed β-adrenoceptors in both lung and heart (Visser et al. 1997). Cardiac uptake of radioactivity was strongly inhibited after ingestion of pindolol (to 39 % of the control value at 60 min postinjection). These pilot studies in humans were performed with noncarrier-added [18F]-fluorocarazolol (∼1 nmol), after it had been shown that fluorocarazolol is not acutely toxic in rodents at doses >10,000-fold higher than were administered to volunteers. For quantification of receptor densities with compartment models, a dual-injection protocol is required involving the administration of a pharmacological dose of the radioligand (∼100 nmol). Such protocols can only be carried out after extensive toxicological screening of the experimental drug. Unfortunately, fluorocarazolol showed a positive Ames test (mutagenicity in bacterial strains) during such examination. Therefore, it was decided to terminate all human studies with [18F]-fluorocarazolol. In contrast to fluorocarazolol, the available toxicological data of carazolol show that the compound is nontoxic even at very high doses. Evaluation in humans should indicate the suitability of [11C]-carazolol as a radiopharmaceutical for clinical PET, although this PET ligand is lipophilic.
11.4.2 PET Imaging of β-Adrenergic Receptors in the Failing Heart
Several factors may induce changes of membrane-bound β-adrenergic receptor density. Major causes are (1) heart failure, (2) myocardial ischemia with or without diabetes, (3) hypertension, and (4) toxic damage. The first study measuring β-adrenoceptor density with [11C]-CGP-12177 PET in patients showed a decreased β-adrenoceptor density in vivo in a group of patients with heart failure due to idiopathic dilated cardiomyopathy (Merlet et al. 1993) (Table 11.2). The [11C]-CGP-12177 PET measurements correlated with β-adrenoceptor density in endomyocardial biopsy. Moreover, these in vivo measurements correlated with functional measurements of β-contractile responsiveness to intracoronary dobutamine infusion. These studies were followed by reports of the group of Camici concerning patients with hypertrophic cardiomyopathy in different phases of disease. Their first report using [11C]-CGP-12177 PET showed a slightly reduced β-adrenoceptor density in patients with primary hypertrophic cardiomyopathy with preserved left ventricular function (Lefroy et al. 1993). These results were in agreement with the hypothesis of an increased sympathetic activity in the heart, which is supported by an elevated myocardial noradrenaline content (Kawai et al. 1983; Tsukamoto et al. 2007) and cardiac spillover of noradrenaline (Brush et al. 1989) in patients with hypertrophic cardiomyopathy. A group with secondary hypertrophic cardiomyopathy due to hypertension and aortic stenosis without heart failure showed a comparable reduction in β-adrenoceptor with [11C]-CGP-12177 PET (Choudhury et al. 1996b). A study in a mixed group of patients with hypertrophic cardiomyopathy with and without signs of heart failure showed a lower β-adrenoceptor density in patients with signs of heart failure and a correlation between β-adrenoceptor density and ventricular function using [11C]-CGP-12177 PET (Choudhury et al. 1996a). From these studies it might be concluded that β-adrenoceptor downregulation precedes clinical heart failure and may be an early clinical marker of left ventricular dysfunction. A study of de Jong and colleagues investigated whether decreased myocardial β-adrenoceptor density in patients with idiopathic dilated cardiomyopathy (IDC) can be estimated using [11C]-CGP-12388 PET (de Jong et al. 2005). They concluded that [11C]-CGP-12388 PET is applicable for the measurement of myocardial β-adrenoceptor density in patients. A highly significant reduction in β-adrenoceptor density was found with a significant difference in β-adrenoceptor density (p < 0.005) between patients with IDC (B max 5.4 ± 1.3 pmol/g) and healthy controls (B max 8.4 ± 1.5 pmol/g). A prospective longitudinal study may yield further evidence to support this finding (de Jong et al. 2005).
Table 11.2
Human (clinical) in vitro B max studies
Disease | B max alternation |
|---|---|
Dilated cardiomyopathy | ↓ |
Myocardial ischemia | ↓ |
Valvular disease | ↓ |
Exercise | ↑ |
Link and colleagues used [11C]-meta-hydroxyephedrine ([11C]-mHED) to image norepinephrine transporter function as an indicator of presynaptic function and [11C]-CGP-12177 to measure global and regional cell surface β-adrenoceptor density as an indicator of postsynaptic function in 19 normal subjects and 9 congestive heart failure patients (Link et al. 2003). Presynaptic, but not postsynaptic, function was significantly different between normals and congestive heart failure patients. Presynaptic function was well matched to postsynaptic function in the normal hearts but significantly different and poorly matched in the congestive heart failure patients studied.
Caldwell and colleagues evaluated in 13 patients with ischemic congestive heart failure and 25 aged-matched healthy volunteers the presynaptic function with [11C]-mHED and the postsynaptic function with [11C]-CGP-12177 (Caldwell et al. 2008) (Fig. 11.2).
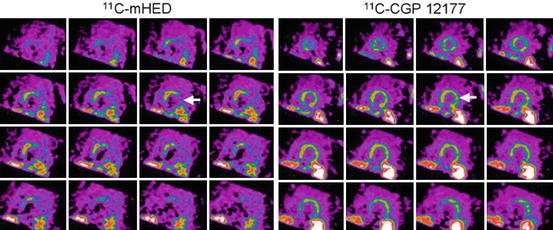

Fig. 11.2
Short-axis PET images of [11C]-mHED (35- to 45 min sum) and [11C]-CGP-12177 (10- to 20 min sum from injection 1) showing left ventricular activity in a chronic congestive heart failure patient. Apical slices are at upper left and basal slices are at lower right of each panel. Arrows indicate extensive mismatch between [11C]-mHED and [11C]-CGP (Caldwell et al. 2008)
Myocardial blood flow was assessed with [15O]-water PET, but global and regional mean blood flow was not different between congestive heart failure and healthy subjects. They found reduced [11C]-mHED and [11C]-CGP-12177 activity in congestive heart failure patients compared with the healthy volunteers and also a mismatch (ratio B max of [11C]-CGP-12177 to [11C]-mHED uptake) between pre- and postsynaptic left ventricular sympathetic function in patients with severe congestive heart failure. After 1.5 year of follow-up, four individuals had an adverse outcome (congestive heart failure death, new or recurrent cardiac arrest or progressive congestive heart failure leading to transplantation). Three of the four patients had mismatch scores >3 times that of the healthy subjects or the congestive heart failure patients without an adverse outcome. Sympathetic signaling in such regions would be more dependent on circulating catecholamines, which are probably lower than those in a normally functioning myoneural junction (Bristow et al. 1992). This decrease could lead to β-adrenoceptor upregulation. However, in patients with dilated cardiomyopathy, [11C]-mHED PET is significantly correlated with the density but not the affinity of uptake-1 sites in the human heart, suggesting either loss of neurons or downregulation of uptake-1 in dilated cardiomyopathy (Ungerer et al. 1998).
After myocardial infarction, LV (left ventricle) remodeling is observed in non-infarcted LV myocardium. LV remodeling is closely associated with systolic heart failure. Myocardial dysfunction is related to the downregulation of cardiac postsynaptic β-adrenoceptors. A recent [11C]-CGP-12177 PET study found out that in the remote non-infarcted region in patients, β-adrenoceptor downregulation was observed, which was related to deterioration of local myocardial systolic function (Ohte et al. 2012).
Furthermore, noradrenaline uptake-1 mechanism and β-adrenoceptor density are reduced in the myocardium of patients with chronic LV dysfunction and evidence of hibernating myocardium (John et al. 2007). The increased sympathetic activity to the heart in these patients is a generalized rather than regional phenomenon which is likely to contributing to the remodeling process of the whole left ventricle rather than playing a causative role in hibernating myocardium.
In patients with syndrome X (Rosen et al. 1996) and asthma (Qing et al. 1997b), i.e., patients with normal left ventricular function, myocardial β-adrenoceptor density was found to be equal to that in normal volunteers, which is in agreement with the general hypothesis that β-adrenoceptor downregulation is only associated with heart failure. Interestingly, myocardial β-adrenoceptor downregulation was also observed in patients with arrhythmogenic right ventricular cardiomyopathy (Schafers et al. 1998). Although these patients have no heart failure, some evidence suggests that their local synaptic catecholamine levels are increased, which apparently causes downregulation of β-adrenoceptor similar to that in patients with heart failure (Wichter et al. 2000). A pharmacological intervention study has been performed in healthy volunteers. This study showed downregulation of pulmonary (Hayes et al. 1996) as well as myocardial β-adrenoceptors (Qing et al. 1997a) after 2 weeks of treatment with a β2-adrenoceptor agonist (albuterol). Naya and colleagues examined if [11C]-CGP-12177 PET could predict improvement of cardiac function by beta-blocker carvedilol treatment in patients with IDC (Naya et al. 2009). They found that myocardial β-adrenoceptor density is more tightly related to improvement of LVEF due to carvedilol than is cardiac contractile reserve as assessed by dobutamine stress echocardiography in patients with IDC. Patients with decreased myocardial β-adrenoceptor have higher resting adrenergic drive, as reflected by plasma norepinephrine, and may receive greater benefit from being treated by anti-adrenergic drugs.
11.5 New Developments
So far, production methods of β-receptor PET ligands were very complex, hampering their widespread use. Because of the potential clinical importance of cardiac β-adrenergic receptor imaging with PET, radiopharmaceuticals should be developed for PET sites without proper production facilities. To this end new radiopharmaceuticals need to be developed which are labeled with [18F] instead of [11C], as [18F] has a longer half-life (110 min) and can be transported to sites within a range of 4 h transport time, which is routinely done on a commercial basis for [18F]-FDG. Beside the disadvantage of the short half-life of carbon-11, CGP-derivatives are non-subtype-selective β-adrenergic receptor ligands. A more selective β-adrenergic receptor ligand characterized with fast plasma clearance and with a high affinity is needed, and β-adrenergic receptor 1 subtype will be the optimal choice in heart studies.
A [18F]-labeled β1-adrenoceptor PET ligand with these optimal properties as mentioned before is needed. Law and colleagues developed and applied a fluoroethoxy derivative of the β1-adrenoceptor antagonist ICI 89406, labeled with fluorine-18 [(S)-[18F]-FICI] (Law et al. 2010) in an animal study. Although in vitro membrane studies showed that (S)-FICI had high affinity and selectivity for β1– adrenoceptors, this study in mice and rats failed to demonstrate high specific binding of (S)-[18F]-F-ICI to myocardial β1-adrenoceptor.
Novel [18F]-fluorination techniques, such as click chemistry, new lead molecules can be synthesized that showed high affinity for β-adrenoceptors. In this click reaction, the bio-orthogonal functional groups alkyne and azide react to form triazoles.
The “click reaction” catalyzed by Cu(I) is a well-established method for rapid and highly efficient synthesis of 1,4-disubstituted triazoles from a wide variety of substrates. Using this method to prepare a β-adrenoceptor ligand, the hydroxyl propylamine moiety (crucial for binding to β-adrenoceptors) can partially be maintained and [18F] is introduced as a novel moiety, hopefully not causing mutagenicity of the carazolol derivatives. A lead compound being a [18F]-fluorinated analog of carazolol, [18F]-FPTC, was produced by a click reaction between a PEGylated [18F]-alkyne and an azidoalcohol derivative of 4-hydroxycarbazol.
A number of studies, either in animals or in human patients, have demonstrated that functionally active autoantibodies targeting the human β1-adrenergic receptor (anti-b1AR-abs) may play an important role in the development and clinical course of progressive cardiac dilatation and failure and increase the risk of developing malignant arrhythmia (Iwata et al. 2001a, b; Magnusson et al. 1994). The presence of these autoantibodies is associated with a markedly worse prognosis in patients with dilated cardiomyopathy (DCM) and ischemic heart disease.
The disadvantage of these anti-b1AR-antibodies is the interaction with the [18F]-labeled β1-adrenoceptor PET ligands which may cause interference with the PET tracer binding to β1-adrenergic receptors.
Future perspectives may include the development of [18F]-labeled subtype-selective β-adrenoceptor ligands to obtain more information about the pathophysiological role of the different subpopulations in vivo. Subtype-selective ligands are being developed for the β1-adrenoceptor as well as the β2-adrenoceptor, but thus far no suitable ligands have been produced and evaluated in clinical studies.
PET has been shown to be a promising technique for the investigation of the role of β-adrenoceptors in cardiac diseases. So far most studies have focused on their role in patients with systolic heart failure (i.e., with a reduced LVEF). However, it is currently unknown whether β-receptor density plays also a role in the development of heart failure and specifically in the development of heart failure with a preserved ejection fraction. The lifetime risk for developing heart failure is 20 % (Lloyd-Jones et al. 2002). Due to the ageing population, the incidence and prevalence of heart failure will increase, not only systolic heart failure but even more heart failure with a preserved ejection fraction (Brouwers et al. 2013). Several studies have identified risk factors for new onset of heart failure, including age, the presence of hypertension, and a history of ischemic heart disease. However, so far the role of β-adrenoreceptor in this is unknown and warrants further investigation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


