21 Permanent Pacemaker and Implantable Cardioverter-Defibrillator Implantation
The approach to cardiac pacemaker implantation has evolved during the past half century.1 From the initial epicardial implants of Senning2 and transvenous implantation by Furman and Schwedel,3 cardiac pacemaker implantation has undergone radical changes not only in the implanted hardware but also in the preoperative planning, anatomic approach, personnel, and implantation facilities. The early trend from the epicardial approach to the simpler transvenous cutdown led to the percutaneous technique developed by Littleford and Spector.4 Previously simple preoperative planning, in particular device selection, has become complex. The pacemaker system, both device and electrodes, must be individualized to the patient’s particular clinical and anatomic situation. The implantation procedure, previously the exclusive domain of the cardiovascular surgeon, has also become the purview of the invasive cardiologist. Similarly, the procedure has undergone a transition from the operating room to the cardiac catheterization laboratory or special procedures room. Except in special instances, the luxury of an anesthesiologist has disappeared, with the implanting physician assuming additional responsibilities. Finally, because of concerns about cost containment, the usual in-hospital postoperative observation period has been dramatically reduced or replaced by an ambulatory approach to pacemaker implantation.
Similarly, since Mirowski et al.5 implanted the first implantable cardioverter-defibrillator (ICD) in 1980, its evolution has been comparable with that of the cardiac pacemaker. The initial epicardial ICD placement with an abdominal pocket has given way to a transvenous approach and a pectoral pocket. The surgery initially performed in the operating room exclusively by a cardiovascular surgeon is now carried out by nonsurgeons in the catheterization or electrophysiology laboratory. Also, protracted hospital stays have been replaced by much shorter hospital stays, even outpatient situations. The once-simple ICD device is now much more complex, offering total arrhythmia control as well as backup dual-chamber rate-adaptive pacing.
 Pacemaker Implantation
Pacemaker Implantation
Personnel
Implanting Physician or Surgeon
It is generally accepted that the pacemaker-implanting physician may be either a thoracic surgeon or an invasive cardiologist.6 At times, the two may even act as a team, with the surgeon isolating the vein and the cardiologist positioning the electrodes. With the current reimbursement structure and the changing economic environment, however, this team approach is rapidly becoming burdensome; in any event, it is frequently unnecessary. Currently, the credentialing for pacemaker implantation procedures poses a dilemma. The trainee in thoracic surgery has ever-diminishing exposure to pacemaker implantation as the procedure becomes more the responsibility of the cardiologist. At the same time, the cardiologist has little or no exposure to proper surgical technique, the use of surgical instruments, and preoperative and postoperative care. Although controversy surrounds the appropriate implantation experience and its length, physicians with limited training and ongoing experience apparently have higher complication rates.7 To remain proficient, the physician should perform a minimum of 12 procedures per year.
In a single-center study of more than 1300 permanent pacemaker implants, Tobin et al.8 reported complications in 4.2% of patients. The economic impact was substantial. Most importantly, there was an inverse relationship between the incidence of acute complication and implanter experience and case volume. Similarly, complications associated with elective generator replacements, revisions, and upgrades have been directly related to operator experience. Harcombe et al.9 found a higher rate of late complications after elective replacements (6.5%) compared with initial implants (1.4%). This higher rate was clearly related to operator inexperience.
There is a definite need for formal training programs specifically designed to teach cardiac pacing.10–12 Such programs should be offered to both cardiologists and surgeons interested in cardiac pacing. The ideal program should be comprehensive and integrated, involving not only all implantations but also follow-up and troubleshooting. To be an effective implanter, the physician must understand the problems of follow-up and troubleshooting. Formal didactic experience and hands-on exposure are necessary. Although a formal, year-long, comprehensive, integrated training program is ideal, consideration of physicians who are out of formal training programs sometimes requires combining more intensive didactic programs with extended, supervised hands-on experience. Training is important for the implantation and nonimplantation aspects of pacing. We see substantially less enthusiasm for the presurgical and postsurgical aspects of pacing. We ardently believe such mastery is crucial to becoming an effective implanter.
Regardless of how physicians have been trained to implant pacemakers, careful review of their training and experience by those granting privileges at the institution will help prevent inadequately trained individuals from performing independent, unsupervised pacemaker implantation. Criteria for adequate training and experience should involve a minimum number of pacemaker procedures, including single-chamber and dual-chamber implantations, lead replacements, pulse generator replacements, and upgrades to dual-chamber from single-chamber systems. Also, some documentable experience in an active pacemaker service clinic should be required.13 An electrophysiology (EP) fellowship is one way of obtaining these skills, and physicians trained as surgeons, pediatricians, radiologists, and cardiologists have access to this training. Credentials can include the EP boards under the jurisdiction of the American Board of Internal Medicine, the Certified Cardiac Device Specialist examination by the International Board of Heart Rhythm Examiners, and cardiothoracic (CT) surgical boards, but only for pacemakers, not ICDs.
Support Personnel
The participation of the manufacturer’s representative as support personnel has always been a subject of debate. This person’s role varies from center to center.14 At one extreme, the representative merely delivers the device and leads to the hospital. At the other extreme, the person is a vital member of the support team, retrieving threshold data, filling out registration forms, and at times, offering technical advice. The latter extreme is particularly true in smaller institutions with less pacemaker activity and in-house support of ICD implantation. A well-trained manufacturer’s representative can be an important member of the support team. An experienced representative dedicated to cardiac pacing and ICD implantation typically has broad experience and a knowledge base in problems unique to the company’s products. Although such a representative of industry can be helpful, this person, no matter how experienced or knowledgeable, should not be considered an acceptable alternative to a knowledgeable, skilled, and experienced physician implanter. If an industrial representative is to be used during implantations for support, hospital approval is advisable.
Implantation Facility and Equipment
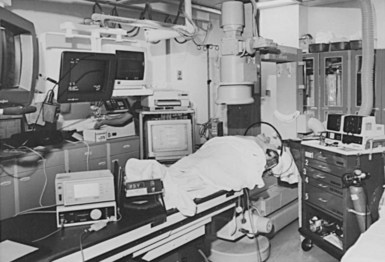
The cardiac catheterization laboratory and special procedures room appear well suited for permanent pacemaker and ICD procedures.15,16 Early concerns about safety and sterility were unfounded, if these issues are appropriately addressed prospectively. Radiologic capabilities are invaluable; high-resolution images, unlimited projections, and angiographic capabilities assist in venous access and electrode placement, as well as in variable image magnification, digital image acquisition, and image imposition techniques and storage. In addition, these facilities tend to be equipped for ready access with all the catheters, guidewires, sheaths, and angiographic materials for special situations. The implantation facility also typically has the most sophisticated physiologic monitoring and recording equipment (Fig. 21-1), offering continuous, surface and endocardial electrical recordings, as well as extensive hemodynamic monitoring capabilities. Again, staffing with qualified cardiovascular nurses and technologists is essential.
The monitoring equipment used for the device procedure is variable. A multichannel electrocardiographic (ECG) recording system is frequently recommended; such systems are able to monitor and record a minimum of three surface electrocardiograms and one intracardiac electrocardiogram.17 From a more practical view, the only requirement is continuous ECG monitoring on an oscilloscope. The ECG pattern need only be clear. Selection of ECG leads should demonstrate adequate atrial and ventricular morphology for defining underlying rhythm, arrhythmias, and atrial and ventricular capture. However, multiple and particularly orthogonal ECG leads are useful to confirm the lead position by ECG morphology.
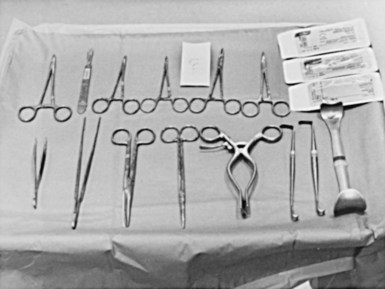
The surgical instruments for a pacemaker procedure are usually found in a “minor surgical” setup (Fig. 21-2). Depending on the institution, the contents of a minor surgery setup can be overwhelming, particularly for the nonsurgeon implanting physician. The rows of unnamed clamps and retractors would suggest the need to enter a major body cavity. Actually, a pacemaker procedure can be performed efficiently with only a few, well-selected instruments,18 and there are many acceptable variations and personal preferences. Problems can occur, however, with the nonsurgeon implanting physician who is unfamiliar with the instruments and their appropriate use.
Box 21-1 lists the contents of an acceptable basic surgical tray for pacemaker implantations. The Gelpi and Weitlaner retractors can be used throughout the procedure for improved visual exposure (see Fig. 21-2). The Senn retractor is used for more delicate retraction of tissue edges; one end is L shaped, and the other has tiny claws. Another useful retractor, the Goulet retractor (see Fig. 21-2), can be replaced with a Richardson retractor and is extremely helpful in retraction when creating the pacemaker pocket. Unlike other large retractors, the smooth, scalloped ends of these retractors are gentle to the tissues while affording a generous area of exposure. Army-Navy retractors can also be helpful for this purpose. Other instruments, such as forceps (with or without “teeth”), hemostats, scissors (tissue and other), needle holders, and clamps, are necessary, but their use does not require explanation here. Proper use and care of the instruments are crucial, and replacement of worn-out instruments is mandatory for avoiding frustration, delays, and suboptimal work.
Device procedures performed in the OR typically benefit from excellent lighting. Multiple high-intensity lamps light the surgical field. However, this is not the case for procedures in the CCL or special procedures room, where lighting is frequently marginal. One solution is a high-intensity headlamp, which is extremely useful when creating the pocket and inspecting for bleeders, particularly when one’s head blocks out other light (Fig. 21-3). Using the headlamp can initially be frustrating and requires practice, but once facile, it will become the major light source for creating the pocket. Even in the OR, despite all the lighting, the headlamp can be very helpful.
The electrocautery device can be useful, and some experienced implanters consider it essential to any pacemaker procedure. Its use, however, is controversial.19–21 Historically, using electrocautery equipment for cutting or coagulation during a pacemaker procedure was taboo, with concerns about causing burns at the myocardium-electrode interface, destroying the pulse generator, and damaging the pacemaker leads. The general consensus, however, is that an appropriately grounded electrocautery device is safe when two precautions are taken: (1) active cautery should never touch the exposed proximal pin of the electrode, and (2) use of all electrocautery should cease when the pulse generator is in the surgical field. Cutting with electrocautery expedites pulse generator changes while avoiding the risk of cutting the lead. At times, even in the most experienced hands, a tedious dissection ends with the scalpel or scissors nicking or cutting the electrode insulation. Use of rapid strokes with cautery avoids the buildup of heat, preventing injury to leads. Although experience indicates no important untoward effects on the myocardium if the cautery touches the pulse generator, there is a risk of causing a permanent no-output situation by destroying the pulse generator. This appears particularly true in certain pulse generators or when the battery voltage is well below the replacement indicator. The risk to the patient of a sudden lack of output can be eliminated by placing a temporary pacemaker in pacemaker-dependent patients; this consideration is fundamental to all pacemaker procedures whether or not electrocautery is used.
The pacing system analyzer (PSA) is extremely valuable during pacemaker procedures. PSA circuitry (especially sensing) mimics that of the planned pulse generator and more accurately predicts the performance of the pulse generator, even when stimulators and recorders are available. The early PSAs were simple and designed to measure the pacing and sensing thresholds for single-chamber ventricular pacing. PSAs were unable to perform (or cumbersome performing) the tasks required for atrial and dual-chamber pacing.22 Previously, PSA devices were designed to test both the lead function and the pulse generator. Currently, PSAs can test lead function and usually can adjust the pacing mode so that both the atrial and the ventricular leads can be tested without risking asystole. Modern PSAs can function in any mode and should measure from either chamber, offering a clear digital display as well as extensive programmability. PSAs provide emergency capabilities, including high output, high rate, and often antitachycardia pacing. In addition, hard copy and electronic transfer of data is useful for documentation. An example of a PSA is the Medtronic model 2090 (Fig. 21-4); Table 21-1 summarizes its desirable features. Some of the pacemakers driven by sensors, (e.g., temperature, oxygen) require special additional sensor analysis by a specialized PSA tool. Whether supplied by the institution or the manufacturer, a good PSA is essential.
Figure 21-4 Multifunction Medtronic 2090 pacemaker system analyzer.
(From Barold SS: Modern cardiac pacing. Armonk, NY, 1985, Futura, p 444.)
TABLE 21-1 Operating Features of the Medtronic CareLink Programmer 2090 Pacing System Analyzer (Medtronic)
| Parameter | Range |
|---|---|
| Models | VOO, VVI, AOO, AAI, DOO, DDD, VDD, ODO |
| Lower rate: | |
| AOO, AAI, VOO, VVI, DOO | 30-220 |
| DDD, VDD | 30-110 |
| Upper rate | 80-220 |
| Amplitudes (A and V) | 0.1-10.0 V |
| Pulse width (A and V) | 0.02-1.5 msec |
| AV interval: | |
| Sensed | 20-350 msec |
| Paced | 20-350 msec |
| Rapid atrial stimulation | 200-800 min. (ppm) |
| Atrial refractory | 200-500 msec |
| Ventricular refractory | 250 msec |
| Atrial sensitivity | 0.25-20 mV |
| Ventricular sensitivity | 0.5-20 mV |
| Polarity (A and V) | Unipolar/bipolar |
| Atrial Blanking | |
| After atrial pace | 160-300 msec |
| After atrial sense | 160-300 msec |
| After ventricular pace: | |
| VVI/VOO | 150-350 msec |
| DDD/VDD | 200-220 msec |
| After ventricular sense | 150 msec |
| Ventricular Blanking | |
| After atrial pace | 40 msec |
| After ventricular sense | 125 msec |
| After ventricular pace | 200 msec |
| Measurement Parameters | |
| P-wave amplitude | 0.3-30 mV |
| R-wave amplitude | 0.6-30 mV |
| Impedance (A and V) | 200-2499 |
| 2500-4000 | |
| Slew rate | 0.1-4.0 V/s |
| Pacing current | 0.1-25 Max |
| Special Features | |
| Rapid stimulation | AOO, VOO, DOO modes |
| Rates | 180-800 ppm |
| Emergency pacing | VVI rate at 70 at 10 V and 1.5 msec |
A, Atrium/atrial; AV, atrioventricular; V, ventricle/ventricular.
There never seem to be enough spare parts during a pacemaker procedure. Most manufacturers offer service kits containing splice kits, stylets, lead adapters, wrenches, lubricant, lead caps, wire cutters, and so on (Box 21-2). It is advisable to set up a pacemaker cart stocked with all the supplies likely to be needed. This cart should hold (1) a temporary pacemaker tray that contains the materials for venous insertion, as well as the temporary pulse generator and leads, (2) an assortment of sheath sets, dilators, and guidewires, (3) the service kits from the manufacturers of the most commonly used pacemakers, (4) the equipment for lead retrieval, and (5) if they are used, a supply of polyester (Parsonnet; C. R. Bard) pouches (Fig. 21-5). A designated person should make sure supplies are reordered and up to date. Other, lesser used supplies can be obtained from the OR or central supply facility, such as a Jackson-Pratt drain for managing hematomas (Fig. 21-6) and various-sized Penrose drains for tunneling.
Preoperative Planning
Planning a pacemaker procedure is important if the case is to proceed smoothly, starting with patient evaluation, including symptoms, medications, and associated conditions. The physical examination may demonstrate the effects of bradycardia, including altered vital signs, evidence of cardiac decompensation, and neurologic deficits. Anatomic issues potentially affecting the implant can also be uncovered. A key preoperative consideration is documentation of the bradyarrhythmia, through 12-lead electrocardiogram (ECG), Holter monitor, event recordings, or inhospital critical care unit or telemetry unit monitoring. Supporting laboratory data, such as digitalis levels, thyroid parameters, and blood chemical analysis, provide documentation that the bradycardia is not secondary to another condition. The patient evaluation should substantiate the indications outlined by the American College of Cardiology (ACC), American Heart Association (AHA), and North American Society of Pacing and Electrophysiology (NASPE) joint task force.23 The documentation should be readily available and is usually affixed to the patient’s chart.
Inpatient Versus Outpatient Procedure
There is a trend toward performing pacemaker procedures on an ambulatory basis. The experiences at several centers, in both Europe and the United States, have clearly supported the safety and efficacy of this approach.24,25 Concerns about potential complications continue to be expressed.26–28 Questions about lead selection, the timing of discharge, and the intensity of follow-up are frequently raised. In addition, the economic impact has yet to be fully appreciated. Although more ambulatory pacemaker procedures are being performed, this has not been reflected well in the pacing literature. Since the original reports of Zegelman et al.24 and Belott,25 Haywood et al.29 have reported a randomized controlled study of the feasibility and safety of ambulatory pacemaker procedures. Although the study group was small (50 patients), the results were similar to those of one of the authors (PHB). There was good patient acceptance, no evidence of a higher complication rate, and cost savings of £540 (at that time, about $810 U.S.).
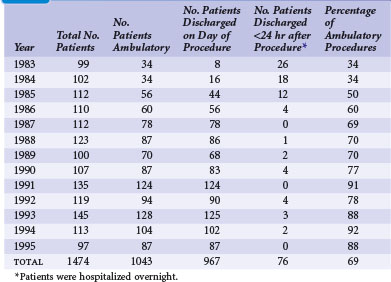
Since the initial report of 181 new pacemaker implants in 1987, our own ambulatory experience continues to be gratifying. During a 13-year span reported in 1996, that experience comprised 1474 pacemaker procedures, 1043 (69%) of which were performed on an ambulatory basis.30 The experience also included pulse generator changes, all of which we have performed on an outpatient basis since 1987. Our experience indicates that 60% to 75% of new pacemaker implantations can be successfully performed as ambulatory procedures (Table 21-2). There have been no additional ambulatory failures, pacemaker-related emergencies, or deaths in the ambulatory procedures. (An ambulatory failure is an implantation that is initiated as an ambulatory procedure, but for which the hospital stay is extended to admitting the patient because of a complication.) The complications encountered in ambulatory cases included one hemothorax detected 2 weeks after discharge, successfully managed by hospitalization and chest tube drainage. Three hematomas were managed on an ambulatory basis with reoperation, control of bleeding, and drain placement. Two small pneumothoraces that did not require chest tubes occurred fortuitously in hospitalized patients who had no planned ambulatory procedure.
These experiences underscore the safety of the ambulatory approach. At present, almost all elective pacemaker procedures (new implantations, electrode repositioning, upgrade procedures, electrode extractions, and pulse generator changes) are done on an ambulatory basis. A simple protocol is used, and the patients often go home 1 to 2 hours after the procedure. They are seen the following day in the pacemaker clinic. Box 21-3 outlines a simple outpatient protocol.
Box 21-3
Outpatient Protocol
In most institutions, patients can remain in the hospital overnight and still be considered outpatients. This practice conforms to the present U.S. Health Care Financing Administration (HCFA) definition of ambulatory surgery for reimbursement in the United States, as follows: “When a patient with a known diagnosis enters a hospital for a specific minor surgical procedure or treatment that is expected to keep him or her in a hospital for only a few hours (less than 24) and this expectation is realized, he or she will be considered an outpatient regardless of the hour of admission, whether or not he or she occupied a bed, and whether or not he or she remained in the hospital past midnight.”31 An important caveat of ambulatory pacemaker procedures is that if there is any doubt or concern about the patient’s well-being, the hospital stay can be extended.
Preoperative Orders
In 2008 the American College of Chest Physicians (ACCP) published evidence-based practice guidelines for the perioperative management of patients receiving antithrombotic therapy,32 including vitamin K antagonists (VKAs) and antiplatelet drugs. ACCP recommends temporary cessation of VKAs and use of perioperative bridging anticoagulation with low-molecular-weight heparin (LMWH) and unfractionated heparin (UFH) for patients at moderate to high risk for thromboembolism and for those with a mechanical heart valve, atrial fibrillation, or venous thrombosis. There is no mention of uninterrupted VKA therapy. For the patient taking antiplatelet drugs who has bare-metal or drug-eluting stents and requires surgery within 6 weeks of stent placement, uninterrupted antiplatelet therapy is recommended. In patients who require temporary interruption of antiplatelets, treatment is stopped 7 to 10 days before surgery. It is recommended that antiplatelet drugs be resumed approximately 24 hours postoperatively. Frequently with pacemaker and ICD procedures, however, antiplatelet therapy cannot be suspended.
Cost controls and managed care can make this process problematic. In addition, despite vigorous attempts at hemostasis, significant hematomas have resulted from the use of heparin. This problem is anecdotal, but in our general experience, the greatest risks for bleeding complications, hemorrhage, and hematoma occur with the use of heparin or platelet antagonists such as aspirin. Having encountered a patient with a devastating thromboembolic complication caused by withdrawal of warfarin, as well as multiple large hematomas from the use of heparin, one of us (PHB) has chosen to perform pacemaker and ICD procedures with the patient still undergoing anticoagulation with oral warfarin. As a rule, patients taking oral anticoagulants have their international normalized ratio (INR) reduced to about 2. With this policy in effect more than 22 years, there have been no devastating hematomas or thromboembolic events. In a recent 13-year retrospective review of 458 device procedures on patients receiving continuous uninterrupted VKA therapy, there were only eight hematomas and no catastrophic hemorrhages or VKA-related deaths.33 This gratifying experience underscores the safety and cost-effectiveness of continuous uninterrupted VKA therapy, which unfortunately remains unaddressed by current guidelines for cardiac implantable electronic device (CIED) procedures.
We believe that pacemaker and ICD procedures can be performed safely with the patient anticoagulated as previously described. Supporting this approach in a 4-year experience, Goldstein et al.34 found no difference in incidental bleeding complications between patients receiving warfarin and those without anticoagulation. No wound hematomas, blood transfusions, or clinically significant bleeding occurred in any patients receiving warfarin. In a later, large series of patients, Giudici et al.35 further substantiated the safety and efficacy of CIEDs without reversing warfarin therapy.
More recently, Ahmed et al.36 demonstrated that interrupting anticoagulation is associated with increased thromboembolic events, and cessation of VKAs with bridging was associated with a higher rate of pocket hematoma and prolonged hospital stays. The authors concluded that continuous uninterrupted VKA therapy with a therapeutic INR was safe and cost-effective. Thal et al.37 compared the incidence of hematoma formation among patients receiving continuous warfarin, aspirin, and clopidogrel therapy. Hematoma formation was rare, even among anticoagulated patients, although an increased incidence was seen in patients receiving dual-antiplatelet therapy. Dreger et al.38 found CIED procedures to be safe in patients on dual-antiplatelet therapy but recommended the use of a drainage system. In patients requiring CRT, Ghanbari et al.39 found uninterrupted warfarin therapy to be a safe alternative to routine bridging therapy, reducing risk of bleeding and shortening hospital stay. More recently, continuing warfarin in patients with a therapeutic INR was shown to be a safe, cost-effective approach compared with cessation of warfarin and bridging anticoagulation.
A new strategy is developing with the release of dabigatran in the United States. This direct thrombin inhibitor has the advantage of a short half-life and obviates the need for INR tracking. The RE-LY study demonstrated its efficacy and safety for patients with atrial fibrillation in comparison to warfarin.40 At this time, no data are available on the impact of dabigatran on perioperative hematomas or the most appropriate perioperative management. However, witholding the medication for 24 hours before the procedure and restarting 24 hours later seems a reasonable initial approach.
Pacemaker Implantation: General Information
Site Preparation and Draping
Currently, many traditional scrubs have been replaced by either a povidone-iodine or a chlorhexidine and alcohol combination. These preoperative skin preparations have the benefit of a single, rapid application. DuraPrep is iodine povacrylex and isopropyl alcohol; ChloraPrep is 2% chlorhexidine and 70% isopropyl alcohol; both offer rapid-acting broad-spectrum protection. Because alcohol-based antiseptic solutions can act as fuel for surgical fires, the skin preparation must be allowed to dry, strictly observing recommended drying times. In addition, it is important to remove the fuel; surgical fires in the OR can result in patient burns and even death.41
The draping process is a matter of personal preference. One of the authors (DWR) applies a sterile, see-through plastic adhesive drape (impregnated with an iodoform solution) over the entire operative area. The other (PHB) uses one or more sterile plastic drapes with adhesive along one side (Fig. 21-7); the adhesive surface is applied from shoulder to shoulder at the level of the clavicle, which serves to create a sterile barrier from the shoulder level down. Depending on the situation, other barriers can be created. In both cases, the plastic drape is used to optimize sterility.
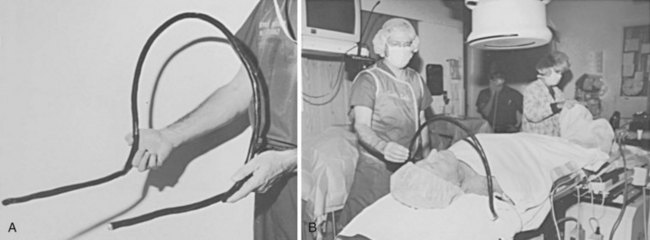
Such an arrangement may not be possible in laboratories, where it interferes with radiographic equipment. Alternatively, the drape may be fixed to the C-arm or image intensifier. This solution is less than optimal; the drapes pull away whenever the C-arm or radiographic table is repositioned, increasing the risks of contamination and breaks in sterile technique. A simple, cost-effective solution consists of a length of common house wire (8/3-gauge Romex) shaped into an arc over the patient’s neck. The ends of the wire are bent at right angles to the arc and tucked under the x-ray table padding at the level of the patient’s shoulders (Fig. 21-8). The weight of the patient’s shoulders supports the wire arc. The wire positioned under the shoulder is checked with fluoroscopy to avoid interference with the radiographic field of view. The house wire is strong enough to keep its shape under the weight of the surgical drape, offering optimal patient comfort and a reliable sterile barrier. There is no interference with the C-arm, and claustrophobia is avoided. The traditional use of a Mayo stand over the patient’s face is problematic because it can cause claustrophobia, makes access to the patient’s airway difficult in an emergency, and may interfere with the x-ray equipment.
Anesthesia, Sedation, and Pain Relief
Most pacemaker procedures are performed with local anesthesia and some form of sedation and pain reliever.42 Local anesthesia alone is inadequate for optimal patient comfort; its effect does not prevent the discomfort associated with creation of the pacemaker pocket. Therefore, the additional combination of a narcotic and sedative is recommended; use of sedation alone is frequently inadequate. The challenge to the physician in charge is to achieve patient comfort without risking oversedation or respiratory depression. If an anesthesiologist or nurse anesthetist is part of the implantation team, patient comfort is usually achieved easily and safely. In this situation, if respiratory depression occurs, the patient can easily be ventilated. When the implanting physician orders the sedation and narcotics, however, the patient must be carefully monitored by the circulating nurse. The medications should be administered slowly.
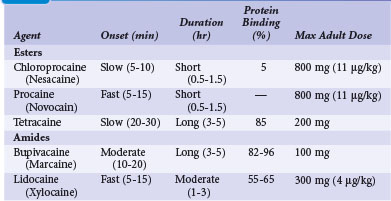
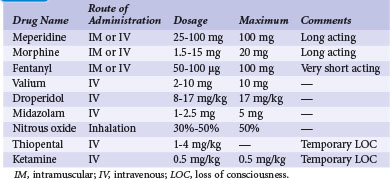
The selection and dose of local anesthetic are also important considerations. A local agent in therapeutic concentration that provides rapid onset of action and sustained duration is desirable. Local agents can be used in combination to achieve the desired effect, such as lidocaine for its rapid onset and bupivacaine for its sustained action. Also, the upper limit of total local anesthetic dose should not be exceeded. Toxic blood levels of local anesthetics can result in profound neurologic abnormalities, including obtundation and seizures. Table 21-3 lists the pharmacologic properties of common local anesthetic agents.
The U.S. Joint Commission on Accreditation of Healthcare Organizations mandates that institutions establish a policy and protocol for patients receiving IV sedation, which would include pacemaker procedures. In essence, the protocol requires formal patient assessment before sedation. Resuscitation equipment must be present at all times in the sedation and recovery areas, and patients undergoing IV sedation must be monitored with pulse oximetry, continuous ECG rhythm monitoring, and automatic blood pressure recordings. Monitoring of the patient should continue for at least 30 minutes after the last IV sedative dose and for at least 90 minutes after intramuscular (IM) sedative administration. There are also strict discharge criteria. Table 21-4 lists common intravenous sedation drug protocols. A North American Society of Pacing and Electrophysiology (NASPE; now Heart Rhythm Society) Expert Consensus developed recommendations and specified minimum training requirements on the use of IV sedation/analgesia by nonanesthesia personnel in patients undergoing arrhythmia-specific diagnostic, therapeutic, and surgical procedures.43
Antibiotic Prophylaxis and Wound Irrigation
The use of prophylactic antibiotics to reduce the incidence of postoperative wound infection in a pacemaker procedure is controversial.44 Importantly, antibiotics are not a substitute for good infection control practices, an adequate surgical environment, and good surgical technique. The use of antibiotics in a pacemaker procedure follows the principle of prophylaxis, in which the risk for infection is low but the morbidity is high.45–47
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree