28 Periprocedural Myocardial Infarction and Embolism Protection Devices
 Introduction
Introduction
Over the last decade, major acute ischemic complications of percutaneous coronary intervention (PCI) have been significantly reduced by advances in pharmacological therapies and interventional devices. Q-wave myocardial infarction (MI), need for emergent bypass grafting, and/or in-hospital mortality have been reduced to 1% to 2% of PCI procedures.1,2 The reduced incidence of these complications can be attributed in large part to the invaluable role of coronary stents in the treatment of abrupt closure and also to the aggressive antiplatelet therapies that have been more commonly utilized over the last decade. This improvement in outcomes is remarkable considering the ever-increasing number and complexity of patients and lesions undergoing PCI today versus 10 or 20 years ago. However, the periprocedural release of cardiac biomarkers is still observed in a considerable proportion of patients undergoing otherwise successful PCI procedures.2,3 Although the etiology and clinical significance of the periprocedural release of cardiac markers are topics for debate, the rise and fall in serum levels of cardiac biomarkers can be described only as a procedure-related acute MI.3
 Definition
Definition
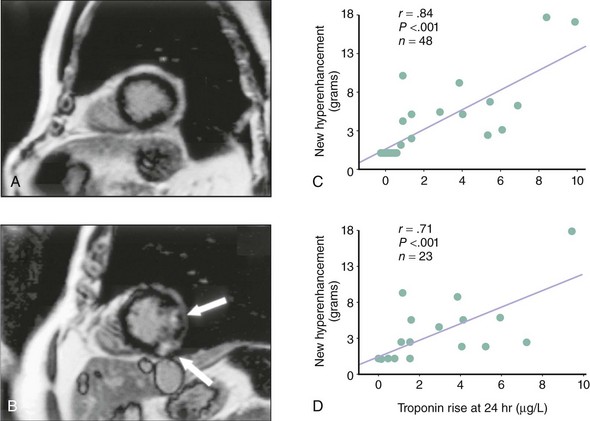
The definition of periprocedural MI (PMI) has been a subject of debate and evolution over the last decade. Traditionally, PMI was defined along the same lines as an acute MI not related to revascularization—that is, it had to meet at least two of three criteria: prolonged chest pain, electrocardiographic Q waves, and a rise in serum markers. Subsequently, numerous publications demonstrated that CK and CK-MB elevations have prognostic implications, even in the absence of pathological Q waves. The cutoff values of CK and/or CK-MB used to define PMI in these studies varied widely. Numerous investigators used the cutoff value of three times the upper limit of normal (ULN) of CK or CK-MB (3x ULN) as the defining threshold of PMI, although it has been traditional to report > 1x ULN, > 5x ULN, and occasionally > 8x ULN values as well.4,5 More contemporary studies have used troponin and myoglobin levels, which are considered to be more sensitive markers for myonecrosis, to define PMI. With the consensus redefinition of acute MI,3 it is now accepted that any elevations of biomarkers above the 99th percentile URL (upper reference limit) after PCI, assuming a normal baseline troponin value, are indicative of a PMI. Pending further data and by arbitrary convention, it is suggested to designate increases more than three times the 99th percentile URL as PCI-related MI (type 4a) in those with elevated troponin levels.6 The definition of PMI is further complicated by the current practice of earlier referral of patients with ST or non-ST segment elevation to the catheterization laboratory. In those situations, the detection of abnormal levels of cardiac markers after PCI may not necessarily be related to the procedure but simply constitute a reflection of the ongoing myonecrosis caused by thrombosis and/or distal embolization that have led to the procedure. In patients with acute coronary syndrome, biomarker levels may rise after an initially negative sample, which commonly coincides with the time when angiography and PCI are performed.7 Such a rise and fall in biomarker levels at the time of PCI is not a “mere” laboratory finding; there is solid evidence of irreversible myocardial injury that correlates with the rise in serum level of the biomarker both qualitatively and quantitatively. In a small study utilizing contrast-enhanced magnetic resonance imaging (MRI) to visualize areas of myonecrosis directly, Riccardi et al. reported evidence of hyperenhancement (equivalent to approximately 2 g of myocardium) in 9 of 9 patients in whom there was minor procedure-related CK-MB elevation (approximately 2x ULN). In the 5 control patients who did not have any CK-MB elevation, there was no MRI evidence of hyperenhancement in the target vessel’s perfusion territory. Importantly, none of the patients in whom hyperenhancement was detected as evidence of myonecrosis developed electrocardiographic Q waves.8 In a more contemporary and rigorous study, 48 patients underwent cardiac MRI before and after PCI to detect newly developed hyperenhancement as evidence of procedure-related myonecrosis.9 Half the patients underwent a third MRI scan at a median of 8 months. Findings were correlated with serum troponin levels recorded 24 hours after the index PCI. All patients were preloaded with clopidogrel and received abciximab at the time of PCI, but the incidence of troponin elevation above ULN was 37% (14 patients). There was evidence of new MRI hyperenhancement in the target vessel’s territory in all 14 patients. In patients with no troponin elevation after PCI, there was no evidence of hyperenhancement on MRI. There was also a linear correlation between the troponin level at 24 hours post-PCI and the mass of newly hyperenhanced myocardium (measured in grams) both on the early post-PCI scan and on the delayed 8-month scan, thus confirming the correlation between periprocedural biomarker release and irreversible myocardial damage (Fig. 28-1).9
In addition to confirming the irreversible nature of the myocardial injury, the location of MRI hyperenhancement in relation to the PCI target segment may give insight into the pathophysiological mechanism underlying PMI. In cases where the hyperenhancement is visualized in proximity of the treated segment, side-branch occlusion is the more likely explanation. But when the myocardial injury/damage is downstream from the treated segment, PMI can best be explained by distal microembolization and adverse platelet and inflammatory reactions in the microcirculation.8,10
 Incidence
Incidence
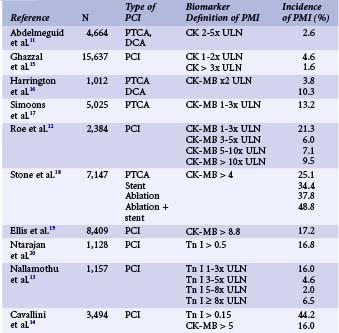
As with its definition, the reported incidence of PMI has varied widely from one published report to another (Table 28-1). This variation can be attributed to several factors: the choice of biomarker assayed, the threshold value used to define PMI, and routine versus clinically driven biomarker assays. When the incidence of PMI is reported for a consecutive series of patients undergoing PCI (irrespective of their clinical condition after the procedure), it is invariably higher than in other series in which biomarkers were assayed only in patients who developed certain symptoms or signs of ischemia. This is the result of detection of a fairly larger proportion of clinically silent events with small-magnitude biomarker release.11 The PCI guidelines update published in 2005 by American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC) experts recommend the routine assay of CK-MB and/or troponin in every patient undergoing PCI 8 to 12 hours after the procedure irrespective of presence or absence of symptoms of MI.2 In nonselected patient series excluding those with initially positive markers, the average incidence of PMI using CK-MB, troponin T, and troponin I > ULN was 23 ± 12 %, 23 ± 12%, and 27 ± 12%, respectively.10 Using a lower biomarker cutoff value to define PMI increases the proportion of patients labeled to have PMI.12,13 Similarly, a higher incidence of PMI (up to 44%) was reported with troponin assays, when compared to studies defining PMI by the traditional CK or CK-MB levels.14 Other important factors that contribute to the heterogeneity of the conclusions of the various published series include the widely disparate baseline and procedural characteristics in the studied populations, inclusion or exclusion of patients with antecedent MI, and the timing of blood sampling.7,10
There may be discrepancies in the reported incidences of PMIs according to the time frame in which various reports were published, the anticoagulation/antiplatelet therapy, and the devices used for PCI. Large Q-wave PMIs were reduced from 2.1% in the BARI trial population to 0.8% in BARI-like patients selected from the more contemporary National Heart, Lung and Blood Institute (BNHLBI) dynamic registry. The reduction was primarily driven by liberal use of stents in the registry, which effectively treated abrupt closure and flow-limiting dissections.21 Additionally, in the EPISTENT randomized controlled trial, use of abciximab reduced Q-wave PMIs during stenting by more than 40% (1.4% vs. 0.8%).22
 Underlying Pathophysiological Mechanisms
Underlying Pathophysiological Mechanisms
MRI of the myocardium in patients who develop biomarker release after PCI has revealed two different types of PMI, according to the distribution of hyperenhancement indicative of acute injury. In the more commonly seen “distal” type of PMI, hyperenhancement is in the distal distribution downstream from the treated segment. In the “proximal” type of PMI, the injury is primarily detected adjacent to the treated segment.8,23 Proximal PMI is usually linked with flow impairment in a side branch arising from the treated segment, whereas the more commonly seen distal PMI results from microvascular obstruction in the distribution of the artery subjected to PCI.
Distal Embolization and Periprocedual Myocardial Infarction
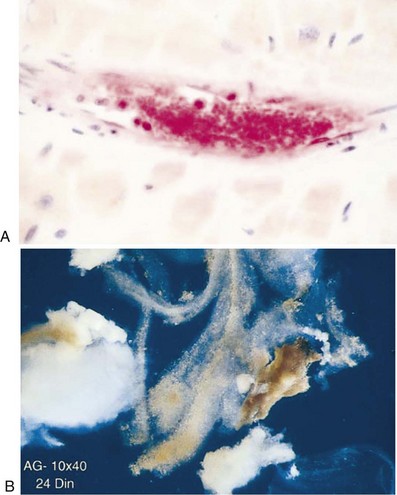
Although distal embolization associated with endothelial injury has been recognized for years, the importance of this phenomenon in relation to PCI was not fully appreciated until the last decade.24 Using filter devices, platelet aggregates have been identified in the distal microcirculation and atherosclerotic debris retrieved from arteries downstream from the site of angioplasty (Fig. 28-2). Clinically, intravascular ultrasound (IVUS) studies have provided further insight into the relationship between embolization of plaque material and PMI. Prati and coworkers examined the relationship between change in plaque volume before and after stenting and the degree of CK-MB release in 54 patients. In patients with unstable angina, there was a more significant reduction in plaque volume. More importantly, however, such reduction significantly correlated with CK-MB release even after adjusting for other variables influencing PMI.25 A more recent and sophisticated analysis by Porto et al. of 62 patients undergoing complex PCI demonstrated a significant association between the change in target lesion plaque area by IVUS and the mass of myonecrosis assessed by hyperenhancement on MRI imaging post-PCI.23 The authors also correlated impaired microvascular flow (TIMI perfusion grade 0 or 1) with MRI evidence of hyperenhancement downstream from the treated segment, hence suggesting that particulate matter from the atherosclerotic plaque disrupted by angioplasty drifts downstream, leading to microvascular obstruction and myonecrosis. The development and clinical utilization of embolism protection devices provided additional evidence of distal embolization, since particulate matter from the atherosclerotic plaque subjected to angioplasty could be collected, measured, and analyzed. The embolized material primarily consists of debris (atherosclerotic plaque and thrombotic elements), with particles ranging in size from ~50 to >600 µm. Neutrophils and macrophages are also identified. Although these devices are more frequently used in the setting of PCI in saphenous vein grafts, distal embolization in routine native vessel PCI is probably just as frequent, with debris that is very similar in quantity and composition.26,27
The Role of the Platelet
Platelet activation plays a critical role in the development and perpetuation of coronary microvascular obstruction following PCI. By definition, the interventional devices used to treat an epicardial stenosis will result in a break in the endothelial surface and release of debris into the coronary bloodstream. The exposed intraplaque contents stimulate platelet activation and aggregation at the site of PCI but probably in the downstream microvasculature as well. Thus the platelet aggregates plugging the microcirculation not only cause mechanical obstruction but also lead to biochemical responses owing to their interaction with the injured endothelium, the neutrophils, and more platelets. The release of vasoactive substances such as serotonin and endothelin-1 from the activated platelets and the injured endothelium lead to intense microvascular vasoconstriction, which accentuates the ischemic injury and resultant myonecrosis.10,24 One study of aspirin resistance emphasized the role of platelet aggregation in the pathophysiology of PMI. Patients deemed to be resistant to aspirin therapy were found to have a significantly higher incidence of PMI, defined as any increase in CK-MB (51.7% vs. 24.6%, P = 0.006).28 The odds of developing PMI in this cohort of 151 patients presenting for nonurgent PCI increased threefold if they were found to be aspirin-resistant prior to the procedure.28
Other Pathophysiological Mechanisms
Distal embolization of plaque debris and platelet activation, with all its local metabolic consequences, probably are the primary mechanisms leading to distal PMI in absence of PCI complications such as side-branch closure or flow-limiting dissections. However, there are other intriguing mechanisms that may interact with embolism and platelet activation. These have been suggested by analysis of coronary sinus blood samples obtained before and after PCI, thus reflecting the local metabolic derangements that result from the intervention. For instance, there is evidence of neutrophil activation in the coronary sinus samples collected post-PCI, as well as an increase in serum levels of C-reactive protein (CRP) and interleukin-6 (IL-6). The increase in the levels of inflammatory markers was associated with post-PCI troponin release.29 This demonstrates a local inflammatory response at the level of the myocardium in response to PCI and suggests a potential contribution to the process of myocyte damage.10,29 Concentration of isoprostanes (stable end products of oxygen free radical mediated-lipid peroxidation) also increased in coronary sinus blood following PCI, which demonstrates an increase in the production of free radicals during PCI.30 The extent to which this inflammatory response and increased oxidative stress contribute to myonecrosis remains unclear.
 Risk Factors Predisposing to Periprocedural Myocardial Infarction
Risk Factors Predisposing to Periprocedural Myocardial Infarction
Clinical Characteristics
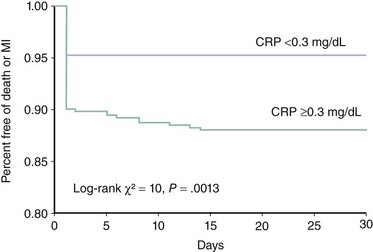
The risk of PMI is significantly increased in patients with evidence of more severe atherosclerotic disease. Multivessel and/or more diffuse coronary artery disease is associated with an approximately 50% increase in the relative risk of developing PMI.12,15,31 IVUS evidence of increased plaque burden is also a risk factor for development of PMI.23,25 This may explain why diabetics are at a higher risk of PMI.32 Interestingly, evidence of advanced noncardiac atherosclerotic disease has been associated with an even higher relative risk of PMI.31 The clinical presentation at the time of PCI may also play a role in determining the risk of PMI and other adverse events during and after the procedure. Patients with acute coronary syndromes are more likely to develop PMI.10 However, studies examining the incidence of PMI in this patient population have been limited by certain methodological difficulties. First, it is difficult and more controversial to define PMI when patients present with elevated markers prior to PCI. Therefore most of the studies on this topic excluded these patients from the analysis. Second, even if patients with elevated markers are excluded, it is conceivable that those with negative markers who were referred to PCI within a few hours of presentation may have been having a spontaneous infarction that was appreciated only after the PCI.10,12 A heightened systemic inflammatory state prior to PCI is also a major predictor of adverse outcomes, including PMI. Most of the evidence supporting this hypothesis is based on correlations between pre-PCI CRP levels and evidence of PMI. A small study of 85 patients with stable angina undergoing PCI demonstrated that PMI (defined by an elevated troponin level) is significantly more frequent in patients with elevated CRP (46% of those with elevated CRP but only 18% of those with a normal CRP developed the complication).33 Chew and colleagues examined the relationship between preprocedural CRP and the adverse events (death and MI) in the first 30 days following PCI in a larger series of 727 consecutive patients. The highest quartile of CRP was predictive of worse outcome (odds ratio [OR] 3.68, confidence interval [CI] 1.5–9.0), and that association persisted even after adjusting for other variables influencing outcome. The event-free survival curves separated within 24 hours and were primarily driven by a reduction in MI, suggesting that patients with elevated CRP are more susceptible to development of PMI (Fig. 28-3).34
Lesion-Related Risk Factors
Saphenous vein graft lesions are notorious for the risk of development of PMI, probably because of the increased incidence of both macro- and microembolization with subsequent slow flow and no reflow. In absence of embolism protection devices, the risk of PMI (defined as CK-MB > 3x ULN) in the contemporary era can be as high as 13.7%. This rate almost doubles if the threshold cutoff value to define PMI is any increase in CK-MB.35The introduction of embolism protection devices in the last few years has significantly reduced the risk of PMI in those patients.35–37 Several lesion characteristics are traditionally associated with a higher risk of PMI, primarily those features suggestive of lesion instability (e.g., eccentricity, irregular contour, visible thrombosis). Complex lesions (ACC/AHA type C) usually contain one or more of those features and are thus associated with a significantly increased risk of PMI.4,11 Lesions involving the ostium of a major side branch are usually among the more complex lesion types and more prone to result in PMI owing to the higher risk of side-branch closure. Other lesion characteristics that confer a higher risk of PMI include those features that suggest a higher plaque burden, such as a multiplicity of lesions, long lesions, and diffusely diseased arteries.
Procedural Complications and Risk of Periprocedural Myocardial Infarction
Side-branch occlusion, flow-limiting dissections, and transient abrupt closure have been the most recognizable procedural complications resulting in relatively large PMIs.4,11,15,38 However, these complications are rare and most detected PMIs follow routine procedures with no obvious angiographic complications.10,24 With the routine use and universal availability of stents, abrupt closure has become quite rare: <1% cases in contemporary PCI.24 The effectiveness of stenting and the availability of potent antiplatelet therapies are probably the primary mechanisms by which large (Q-wave) PMIs and the need for emergent coronary surgery have been reduced by almost 50%.21,39 Abrupt closure or no reflow have not been found to affect outcome when treated promptly, with no subsequent PMI.38 Side-branch occlusion (SBO) has been and remains the most common angiographcially recognizable procedural complication resulting in PMI.11,15 Unlike abrupt closure, the incidence of SBO has not decreased with the routine use of coronary stenting. In fact, with increasing stent use, SBO has become the most likely cause of acute occlusion during PCI.40 Major SBOs can be associated with large (possibly Q-wave) infarctions, but even smaller branch occlusions have been associated with evidence of small areas of MRI hyperenhancement, diagnostic of small areas of PMI. The distribution of hyperenhancement in these cases is different from that seen with distal embolization downstream of the target lesion for PCI. With SBO, hyperenhancement is “adjacent” rather than “distal” to the location of the PCI. The likelihood of developing a new hyperenhancement increases 16-fold when an SBO can be recognized angiographically.23 There have been some intriguing observations regarding SBO and PMI in the era of PCI of complex lesions using drug-eluting stents. In the TAXUS V trial, which randomized 1,172 patients to receive either paclitaxel-eluting or bare metal stents, complex lesion subsets (more than 35% type C) were treated in both groups and >30% of patients received more than one stent. In the subgroup of patients receiving multiple stents, the incidence of 30-day MI was significantly higher with paclitaxel-eluting stents (8.3% vs. 3.3%, P = 0.047). Core laboratory angiographic analysis of this patient subset revealed a significantly higher incidence of side-branch compromise or occlusion with paclitaxel-eluting stents than with bare metal stents (42.6% vs. 30.6%, P = 0.03), resulting in a higher incidence of < TIMI 3 flow in the paclitaxel-eluting stent group. Why paclitaxel-eluting stents are associated with more side-branch compromise and subsequent PMI remains unclear. Possible explanations include the increasing thickness of the stent struts caused by the drug-eluting polymer, increased platelet deposition, and/or paclitaxel-induced spasm.41 A comparison of paclitaxel-eluting and everolimus-eluting stents demonstrates the importance of the strut and polymer thickness. A post-hoc analysis of the SPIRIT III randomized trial compared the incidence of PMI in 113 patients receiving the thinner strut/thinner polymer everolimus-eluting stent with that in 63 patients receiving the paclitaxel-eluting stent, in whom a small side branch was “jailed” by the deployed stent. PMI, defined as any increase in CK-MB above ULN, was much lower in the everolimus group (9.0% vs. 29.7%, P = 0.01).42
Risk of Periprocedural Myocardial Infarction by Interventional Device
Some of the earliest investigations sparking interest in PMI and its significance were in the context of comparing newer interventional devices with standard balloon angioplasty. Data from the CAVEAT-I trial demonstrated that directional atherectomy (DCA) was associated with more abrupt closure, evidence of PMI, and a subsequently higher rate of clinical adverse events compared with balloon angioplasty.16,43 These findings were confirmed in the BOAT trial, in which a more refined technique of DCA was supposed to demonstrate its superiority to PTCA. However, the incidence of PMI was still significantly higher with DCA than with balloon angioplasty (16% vs. 6%).44 DCA is associated with more distal embolization, particularly in saphenous vein graft interventions.45 There is also evidence of a higher degree of platelet activation with DCA,46 with its subsequent mechanical obstruction as well as thrombotic and inflammatory responses in the downstream microcirculation. Similarly, rotational atherectomy is associated with more platelet activation and distal embolization of plaque debris than balloon angioplasty owing to its mechanism of action.47 Although the routine use of coronary stents has dramatically reduced the incidence of most PCI complications (abrupt closure, flow-limiting dissections, need for emergent bypass surgery, and restenosis), stenting increases the incidence of PMI compared with balloon angioplasty, with a relative risk increase of ~20%.10,24,48 In patients undergoing PCI of the left anterior descending artery randomly assigned to balloon angioplasty or stenting, there was evidence of a higher degree of platelet and neutrophil surface activation after stenting.49 High-pressure inflations aiming to overexpand stents and reduce restenosis can actually lead to higher CK-MB levels. In a study of approximately 1,000 patients undergoing IVUS-guided stenting, the incidence of PMI (defined as CK-MB 3x ULN) was 16%, 18%, and 25% in three groups of patients in whom the final stent-to-reference lumen area was <70%, 70% to 100%, and >100%, respectively.50
 Prognostic Implications of Periprocedural Myocardial Infarction
Prognostic Implications of Periprocedural Myocardial Infarction
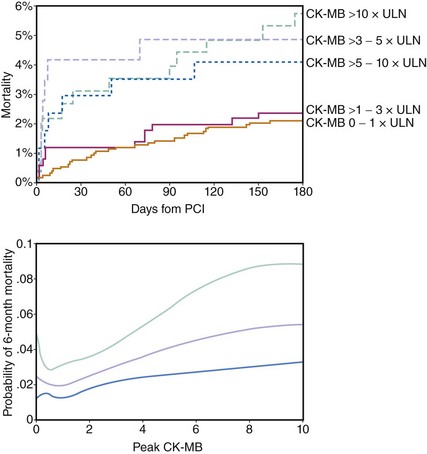
Although there is much controversy about the definition and prevalence of PMI with everyday PCI, there is no dispute that significant PMI is associated with an increased mortality risk. There remains controversy about the pathophysiological mechanisms underlying this association as well as the definition and the size of PMI that would confer such increased risk. However, there is convincing evidence that any PMI is associated with some degree of increased risk of death, particularly with longer follow-up. The pioneering work of Abdelmeguid and coworkers had demonstrated that CK and CK-MB elevation post-PCI (primarily balloon angioplasty and DCA in this report) are associated with an approximately 30% relative increase in 3-year mortality.11 Three-year follow-up of the EPIC trial patients (undergoing angioplasty and DCA as well) revealed an incremental long-term risk of death with increasing degrees of PMI. Among the 2,001 patients enrolled in the trial, the mortality risk increased from 7.3% in those with no CK elevation to 13.1% when CK was > x3 ULN and again to 16.5% with CK > 10x ULN.51 In the EPISTENT trial, in which stenting was routinely employed in two-thirds of the patients, the 1-year mortality doubled between patients with no or minimal PMI (CK-MB > 1x ULN) to those with CK-MB > 3 to 10x ULN (1.5% vs. 3.4%).22 Subsequently, similar conclusions were reached by examining outcomes of patients enrolled in PCI clinical trials and large-scale single-center patient registries (Table 28-1).12,15,16,19,22,52 Although the association between PMI and mortality has not been disputed, the mechanisms that can explain this association are not clear. The magnitude of myonecrosis is limited, but it may provide a nidus for arrhythmogenesis. On the other hand, it has been suggested that the association between PMI and late mortality merely represents a reflection of increased risk in a group of patients with more advanced disease.7 The latter hypothesis can be criticized by the fact that aggressive preprocedural platelet inhibition (by thienopyridine pretreatment or intravenous GP IIb/IIIa inhibitors) reduces PMI and the subsequent risk of death and adverse events, suggesting that this is a modifiable risk. The threshold above which a PMI is considered prognostically significant has been a subject of some debate. It has been traditional to consider a PMI when the CK-MB is > 3x ULN, although the recent PCI guidelines suggest CK-MB > 5x ULN should be the threshold for defining a PMI.2 In the large series of Ghazzal and colleagues, minor elevation of total CK (< 3x ULN) did not confer a statistically significant increase in risk of late mortality.15 Brener and coworkers suggested that only massive CK-MB release (> 10x ULN) predicts an increased risk of death over a 3-year period.53 However, there has been strong evidence that smaller CK-MB elevations are associated with increasing risk of death. Abdelmeguid and colleagues examined that question specifically and concluded that any increase in CK-MB above normal limits confers some degree of risk.11 In later meta-analysis, any increase in CK-MB, even < 3x ULN, was associated with a statistically significant increase in the risk of death (OR 1.5). Patients with CK-MB levels between 3x and 5x ULN and those with levels > 5x ULN had an even higher relative risk of dying over the 3-year follow up.54 Similarly, in the large metanalysis of Roe and colleagues, an increased mortality risk was associated with increasing CK-MB expressed as a continuous variable—that is, with no specific thresholds above or below which the risk changes (Fig. 28-4).12 Frequently, very large PMIs (i.e., with CK-MB levels > 8x-10x ULN) are associated with significant complications or an unsuccessful procedural result. The association between PMI and mortality has been attributed to the impact of unsuccessful procedures on mortality and not to an independent effect. In a study of approximately 6,000 patients, the incidence of PMI was three times more frequent when the procedure was unsuccessful, and the size of the infarction was also significantly larger. After adjusting for the success of the procedure (defined as residual stenosis <50%; achievement of TIMI 3 flow; and absence of significant residual dissection, need for urgent revascularization, or stent thrombosis within 24 hours), the presence or absence of PMI was not statistically related to 1-year mortality.55 However, the study examined only 1-year mortality, and in many investigations examining PMI, the effect on mortality was observed only with longer-term follow-up. In addition, the initial studies by Abdelmeguid et al., which established a relationship between PMI and death, excluded unsuccessful procedural results from the analyses.4,11 In the recent CK-MB and PCI study examining the significance of post-PCI troponin elevation, unsuccessful procedures doubled the odds of 2-year mortality, yet the effect of post-PCI CK-MB levels remained a strong and significant predictor of mortality.14 The association between mortality and PMI defined by elevated serum troponin levels is less robust. The updated PCI guidelines propose that a PMI becomes clinically significant if the troponin level is > 5x ULN.2 In a study of 1,157 patients (>77% receiving stents), 1-year mortality risk increased only in the group of patients with troponin I levels ≥ 8x ULN (≥16 ng/mL).13 However, in the largest multicenter prospective study (almost 3,500 patients) addressing the significance of post-PCI troponin levels, there was no statistically significant association between troponin I elevation and 2-year mortality. As expected, the incidence of troponin I elevation post-PCI was significantly higher than that of CK-MB, indicating the higher sensitivity of that marker in detecting myonecrosis. Yet, this high sensitivity appears to reduce the ability of troponin elevation to predict prognosis.14 A contemporary analysis on the prognostic significance of PMI in patients from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial suggested that PMI is a marker of baseline risk, atherosclerosis burden, and procedural complexity but in most cases does not appear to have independent prognostic significance.56
 Prevention and Management of Periprocedural Myocardial Infarction
Prevention and Management of Periprocedural Myocardial Infarction
Intravenous Glycoprotein IIb/IIIa Platelet Inhibitors
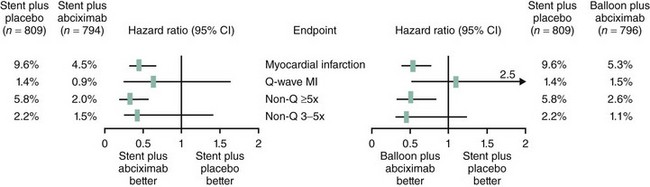
Periprocedural utilization of these agents provides immediate and near complete inhibition of platelet aggregation. The intravenous administration of the appropriate doses and the targeting of the final common receptor for the aggregation process (the IIb/IIIa receptor) ensure both an extremely high bioavailability and a very predictable and complete response. Abciximab, the prototype of this class of antiplatelet agent, has been shown in multiple clinical trials to reduce the incidence of post-PCI myonecrosis. In the seminal trials (EPIC, EPILOG, and EPISTENT), the cutoff threshold for defining PMI was CKMB 3x ULN. The relative risk reduction in MI at 30 days ranged between 40% and 60%, with the curves separating as early as the first day, thus indicating a significant reduction in PMI (Fig. 28-5).21,40 In the CAPTURE trial, patients with refractory unstable angina were randomized to receive abciximab or placebo many hours before PCI and during the procedure. In this trial, the incidence of PMI was reduced by >50% in the abciximab arm (2.6% vs. 5.5%, P = 0.009).57 With rotational atherectomy, which consistently results in distal microembolization, abciximab bolus and infusion demonstrated a significant advantage over anticoagulation alone in reducing the rise in postprocedural CK and CK-MB.47 Similar effects have been demonstrated with the synthetic small-molecule IIb/IIIa inhibitors eptifibatide and tirofiban. The impact of eptifibatide on PMI was significantly greater when the dosing regimen was adjusted from one bolus in the PURSUIT-PCI trial to the double-bolus regimen followed in the ESPRIT trial, thus emphasizing the importance of the near complete inhibition that is required if an impact on distal embolization is to be expected. In the PURSUIT trial, the incidence of PMI was reduced by 25%, whereas the reduction with the double-bolus regimen was 40%. In both trials, PMI was defined as CKMB > 3x ULN and the incidence of PMI in the placebo group was very similar in both trials: ~9%.58,59 Similarly, in the RESTORE trial examining the role of tirofiban in patients with acute coronary syndromes, there was a small but significant reduction in PMI at the 48-hour mark. However, there was no statistically significant difference in the incidence of the primary endpoint (30-day death, MI, or revascularization for recurrent ischemia, use of stents for threatened or abrupt closure) between tirofiban and placebo.60 With a higher-dose tirofiban bolus, a small study of 202 high-risk patients undergoing PCI demonstrated that PMI defined by troponin rise decreased by ~34% and average CKMB level (expressed in absolute units) was reduced by >50%.61