Chapter 63 Peripheral Arterial Occlusive Disease
Epidemiology
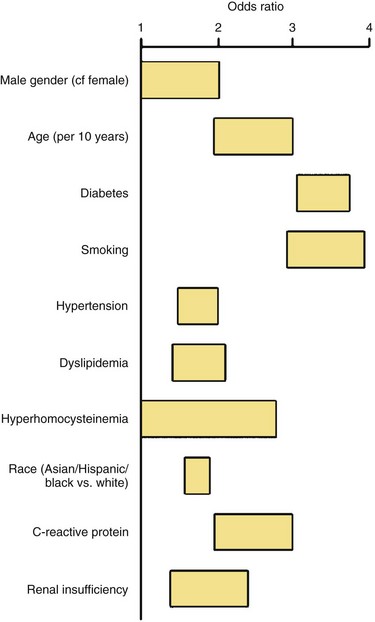
PAD is more prevalent in nonwhite populations, and this is not completely explained by an increased incidence of comorbid diseases.1 An ankle-brachial index (ABI) less than 0.90 is almost twice as common in non-Hispanic blacks than whites. Risk is increased in smokers and in patients with hypertension (HTN), dyslipidemia, hypercoagulable states, renal insufficiency, and diabetes mellitus (DM) (Fig. 63-1). The prevalence of PAD is strikingly higher in a younger diabetic population, affecting one in three diabetics older than 50 years. Diagnosis is critical, because people with PAD have a risk of heart attack or stroke four to five times higher than the age-matched population (Fig. 63-2). The risk of PAD also increases in individuals who older than 50 years, male, obese, or with a family history of vascular disease, heart attack, or stroke. Other risk factors that are being studied include levels of various inflammatory mediators, such as C-reactive protein and homocysteine.
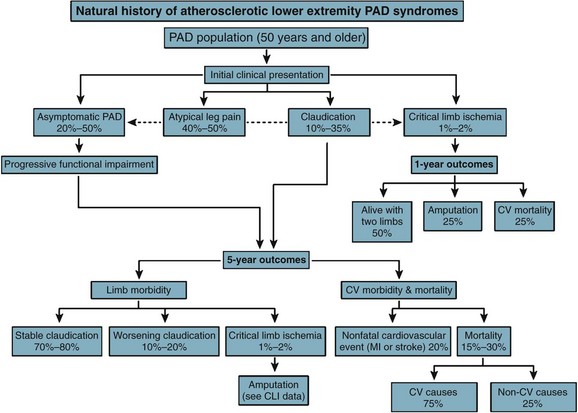
FIGURE 63-2 Outcomes of atherosclerotic peripheral arterial disease at 5 years.
(From Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines: ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease [lower extremity, renal, mesenteric, and abdominal aortic]: A collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines [writing committee to develop guidelines for the management of patients with peripheral arterial disease]—summary of recommendations. J Vasc Interv Radiol 17:1383–1397, 2006.)
Basic Science of Vascular Disease
Vascular Wall Microanatomy
The arterial wall consists of three concentric layers:
Evaluating And Treating The Patient With Peripheral Arterial Disease
Chronic Arterial Insufficiency
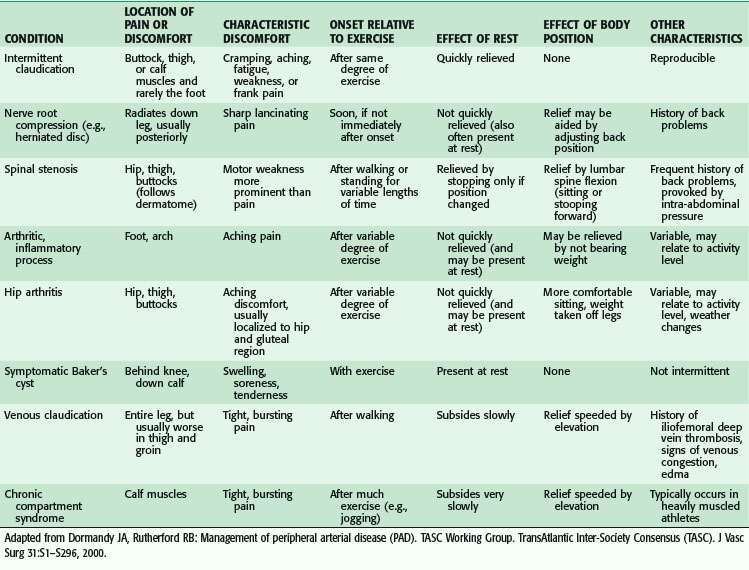
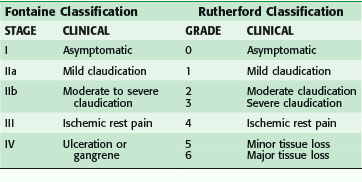
The clinical presentation ranges from asymptomatic to gangrenous tissue loss. Intermittent claudication is a common presentation in the outpatient setting, and usually signifies mild to moderate vascular occlusive disease. Classically, pain occurs with activity or ambulation and is relieved with rest. Because of the frequency of superficial femoral arterial disease, the usual location of the pain is in the calf, but claudication may also involve the thighs or the buttocks because the arterial disease may be located in the aorto-iliac segment. The arterial disease is usually one level above the symptomatic muscle group. The differential diagnosis of leg pain is broad and the treatment modalities are equally disparate. Table 63-1 outlines an approach to the differential diagnosis of claudication.
Patients who are limited in ambulation because of arthritis, severe lung disease, or heart failure, or who are diabetic with neuropathy, may not experience leg pain and may present initially with advanced disease. Worsening perfusion leads to critical limb ischemia (CLI), which may be manifested by rest pain. This is described as pain that occurs at rest; it may wake the patient from sleep. CLI patients present also with tissue loss with ulceration or nonhealing wounds of the foot. This usually in the dorsum of the foot, relieved with dangling the leg over the edge of the bed. Patient may also have tissue loss with ulcerations or nonhealing wounds of the foot (Table 63-2).
Initial evaluation must include a detailed medical history of comorbid conditions. In addition to coronary artery disease (CAD), carotid artery stenosis (CAS), and prior stroke, risk factors for atherosclerosis (e.g., diabetes, hypertension, dyslipidemia, tobacco abuse, hyperhomocysteinemia) should be queried and their level of optimization understood. Because medical management is a cornerstone of vascular therapy, a review of the patient’s medications is imperative, with attention to the potential need for antiplatelet agents, beta blockers, angiotensin-converting enzyme (ACE) inhibitors, and statins as a matter of course. Previous exposure to heparin, protamine, and NPH insulin (neutral protamine Hagedorn)2 should be noted. Allergies to contrast agents or iodine should be documented.
Physiologic Testing and Imaging
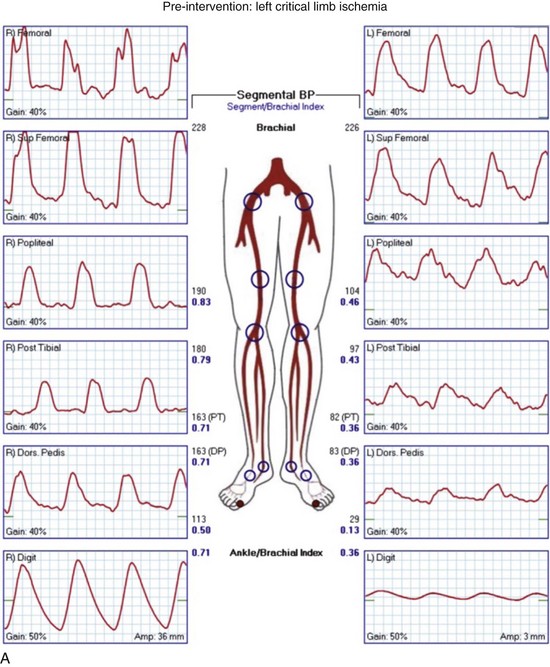
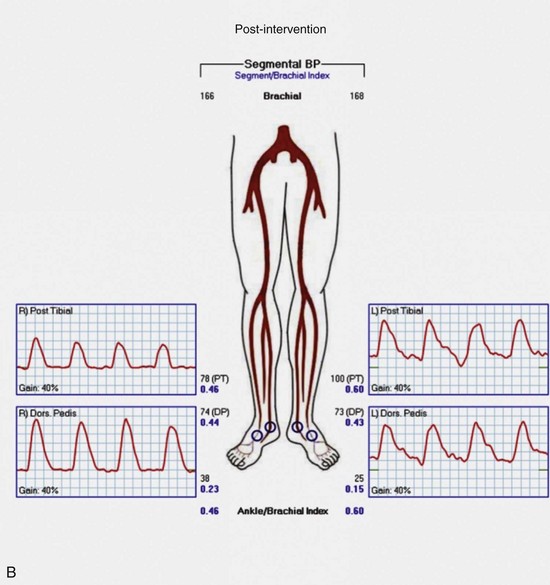
Regardless of plans for intervention, it is recommended that asymptomatic patients at risk for PAD and those with symptoms undergo ABI testing. This examination can be performed simply with a manual blood pressure cuff at the ankle and a continuous wave Doppler probe. With the patient in a supine position, after several minutes of rest to allow limb pressure to return to baseline, the cuff is inflated at the ankle, with the Doppler probe held at the location of the distal DP or PT signal. The systolic pressure is recorded as the pressure in the cuff when the Doppler signal returns. This process can be performed with multiple cuffs allowing for segmental pressure determination (Fig. 63-3), which is helpful in localizing the level of the obstructing lesion. The ABI for a limb is calculated using the higher of the two ankle pressures divided by the higher of the two brachial pressures (Tables 63-3 and 63-4). Patients with an ABI of 0.90 or less have a three- to sixfold increased risk of cardiovascular mortality.
Table 63-3 Clinical Correlation of Different Levels of ABI*
| ABI | PRESENTATION |
|---|---|
| 1.11 ± 0.10 | Normal |
| 0.59 ± 0.15 | Intermittent claudication |
| 0.26 ± 0.13 | Ischemic rest pain |
| 0.05 ± 0.08 | Tissue loss |
* The diagnosis of PAD is given to ABI <0.9. ABI >1.3 is interpreted as abnormal because of incompressible tibial arteries, frequently seen in diabetes and end-stage renal failure. Example: The ABI is calculated using the higher of the two ankle pressures (as indicative of limb perfusion), and the higher brachial pressure (as indicative of systemic pressure). In this example, the left and right ABI values are both 0.30.
From Moneta GL, Zaccardi MJ, Olmsted KA: Lower extremity arterial occlusive disease. In Zierler RE, editor: Strandness’s duplex scanning in vascular disorders, ed 4, Philadelphia, 2010, Lippincott Williams & Wilkins, Wolters Kluwer Health, pp 133–147.
| PARAMETER | RIGHT | LEFT |
|---|---|---|
| Brachial blood pressure | 150 mm Hg | 100 mm Hg |
| Dorsalis pedis | 50 mm Hg | 25 mm Hg |
| Posterior tibia | 25 mm Hg | 50 mm Hg |
| ABI | 0.30 | 0.30 |
Continuous wave Doppler analog waveforms can be obtained along with the segmental pressures. PPG uses an infrared light-emitting source and a photosensor; it is based on the principle that red light is decreased with increased blood flow in tissues to generate a pressure and waveform within the digit. The data generated from these studies should include bilateral brachial artery, high thigh, low thigh, calf, DP, PT, and toe pressures with waveforms (Fig. 63-4). A decrease in pressure of 20 to 30 mm Hg between adjacent segments is indicative of a significant lesion. The normal Doppler arterial waveform demonstrates triphasic flow with a sharp systolic upstroke, reversal of flow in early diastole from vessel compliance, and low-amplitude forward flow throughout diastole. With obstructive disease, the initial feature lost is the reversal of the flow component, leading to multiphasic (previously called biphasic) flow. Severe disease leads to blunting of the arterial waveform, with decreased amplitude and decreased slope of the upstroke. With worsening symptoms, there is increased diastolic flow, resulting in monophasic flow. A change in waveform can be interpreted, along with a change in pressure, as indicative of disease at that level. Limitations of ABI and segmental pressure determinations include mural calcification, such as seen in DM and ESRD, leading to elevated pressures that do not accurately reflect intra-arterial perfusion pressure. With a noncompressible vessel, a TBI higher than 0.70 with an absolute digit pressure higher than 50 mm Hg, with a normal waveform, is indicative of preserved flow because digit arteries are relatively resistant to the intramural calcification. The high thigh pressure cannot always distinguish among common iliac, external iliac or common femoral disease. A proximal stenosis can decrease flow to the extent that accuracy is lost when interpreting gradients downstream.
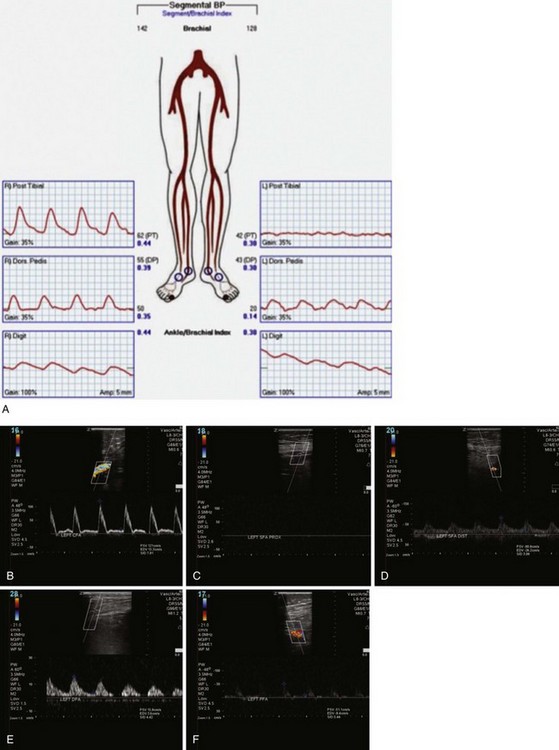
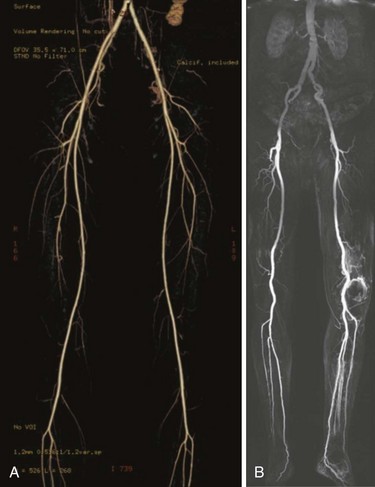
Arterial duplex ultrasonography provides B mode (gray scale) imaging, pulsed Doppler spectral waveforms, and color flow data for analysis and, in experienced hands, can provide sensitive and specific information about the abdominal aorta and visceral, renal, iliac, and distal limb vessels. Peak systolic velocities (PSVs) and end-diastolic velocities are recorded. Waveforms are generated and analyzed. Color flow is useful for demonstrating patent vessels in very low-flow states and for distinguishing antegrade from retrograde flow. As in continuous flow Doppler analysis, a change in waveform from triphasic to monophasic, or an increase in PSV followed by a drop in velocity, indicates a hemodynamically significant lesion. A ratio of the PSV within the stenosis to the PSV of the proximal normal segment of 2.0 or more correlates with a stenosis of 50% or more. Visualization of intra-abdominal segments requires the patient to be fasting prior to the examination to eliminate bowel gas; studies can be limited by body habitus. Severe calcification of the distal vessels can impede imaging of flow (Figs. 63-5 and 63-6).
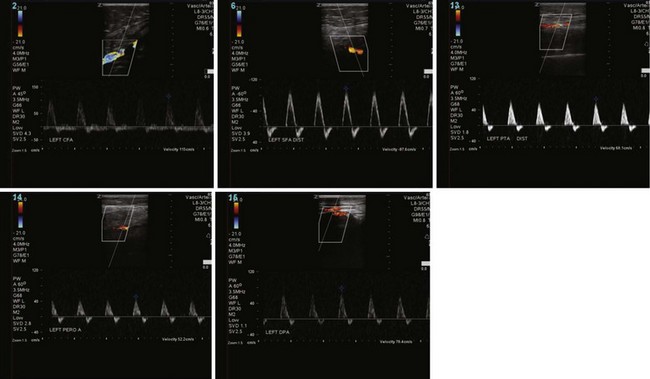
FIGURE 63-6 Postintervention on the same patient as Figure 63-5 shows intact flow throughout SFA and improved distal flow.
Imaging Studies
Angiography
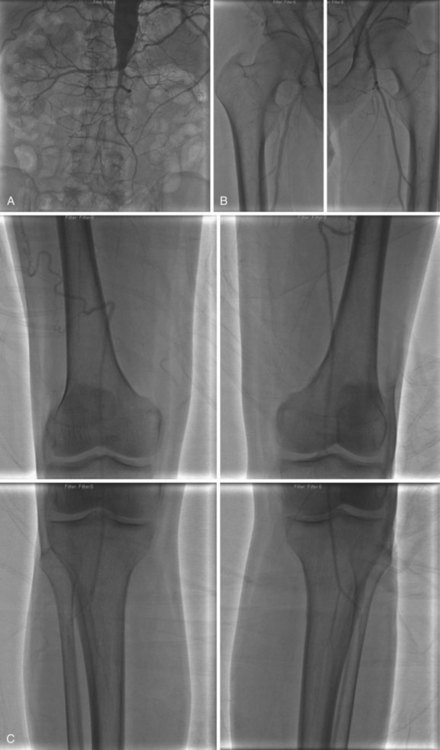
Access is usually via the contralateral common femoral or left brachial artery. A complete diagnostic study is performed in four steps: (1) abdominal aortography, with a multiside hole catheter placed at the level of the diaphragm, imaging the abdominal aorta, celiac artery, superior mesenteric artery (SMA), inferior mesenteric artery (IMA), and aortic bifurcation; (2) pelvic angiography with a multiside hole catheter at the aortic bifurcation, imaging the bilateral common iliac, hypogastric, and external iliac arteries, common femoral arteries, and proximal superficial femoral (SFA) and profunda femoris artery (Fig. 63-7). (3) The contralateral common femoral artery is then selected using an end-hole catheter, and images of the contralateral SFA, profunda, popliteal, tibial, and pedal vessels are obtained in one to three low-bolus runs. (4) The access sheath is then pulled back to the level of the distal ipsilateral external iliac artery to image the ipsilateral limb. Trans-stenotic pressure gradients and multiplanar images can clarify the significance of an ambiguous lesion. Complete assessment of the aortic and iliac inflow and bilateral lower extremities requires 75 to 100 mL of contrast.
Computed Tomography Angiography
The widespread use of multidetector row CT scanners has improved the speed, volume coverage, and slice thickness of images so that a single contrast bolus can be imaged as it passes through the arterial system. One advantage of CTA (Fig 63-8A) is the depiction of the entire vessel, with the ability to appreciate thrombus and calcification; arteriography typically characterizes only the lumen of the artery. Thin slices of 0.625 mm allows for three-dimensional reconstructions and multiplanar reformatting that is not routinely achieved with conventional arteriography. CTA disadvantages are similar to those of arteriography, with the potential for complications from the use of iodinated contrast agents and significant accumulation of radiation exposure.
Magnetic Resonance Angiography
Advocates of contrast-enhanced MRA with gadolinium (see Fig. 63-8B) report a high sensitivity and specificity of this modality for demonstrating the degree of stenosis and lesion length, and even superiority in identifying distal target vessels when compared with conventional arteriography.3 Disadvantages of MRA technology include the need for patient cooperation, patient discomfort, longer studies, expense, contraindications with certain metallic implants, and renal toxicity reported with use of the contrast agent, Gadolinium. Its use is contraindicated in renal disease because of the risk of nephrogenic systemic fibrosis. This is a rare complication associated with the administration of gadolinium-based agents to patients with renal failure or renal insufficiency having a glomerular filtration rate 30 mL/min or lower.4 Patients develop fibrosed nodules of the skin, eyes, and joints. Severe contracture limiting movement or involvement of the heart, liver, and lungs has been described.
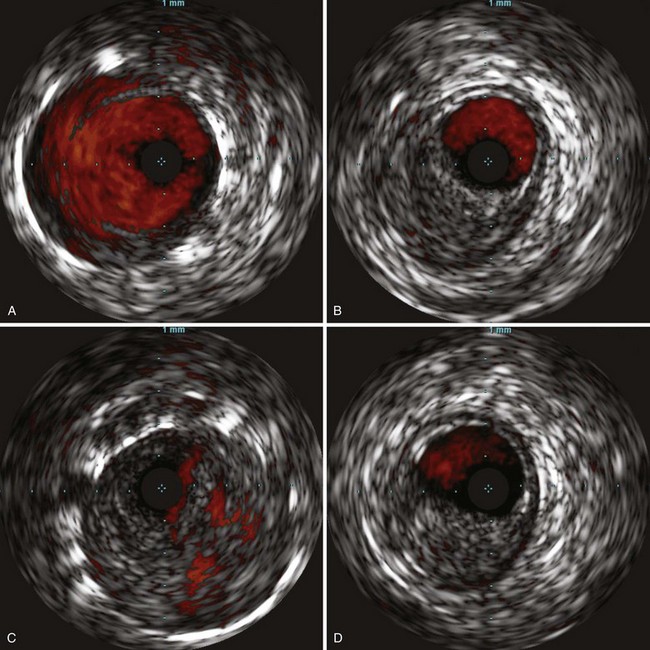
Intravascular Ultrasound
With improvements in high-frequency smaller transducers, the use of catheter-based intravascular ultrasound IVUS (Fig. 63-9) has increased. IVUS provides a transverse, 360-degree image of the lumen of the vessel to be imaged throughout its length and provides qualitative data about the wall anatomy. It has been used in peripheral interventions for opening chronic total occlusions (CTOs) and has been instrumental in the endovascular treatment of aortic dissection. As a diagnostic tool, adjuncts such as color flow Doppler enable the delineation between flow and thrombus, whereas virtual histology, in which color is assigned to plaque components of fibrous, fibrofatty, calcified, and necrotic lipid core densities, has been shown to correlate well with actual histology in assessment of coronary5 and carotid arteries disease.6 The use of IVUS, however, increases the length of procedures and its expense limits its applicability.
Treatment
Medical Treatment
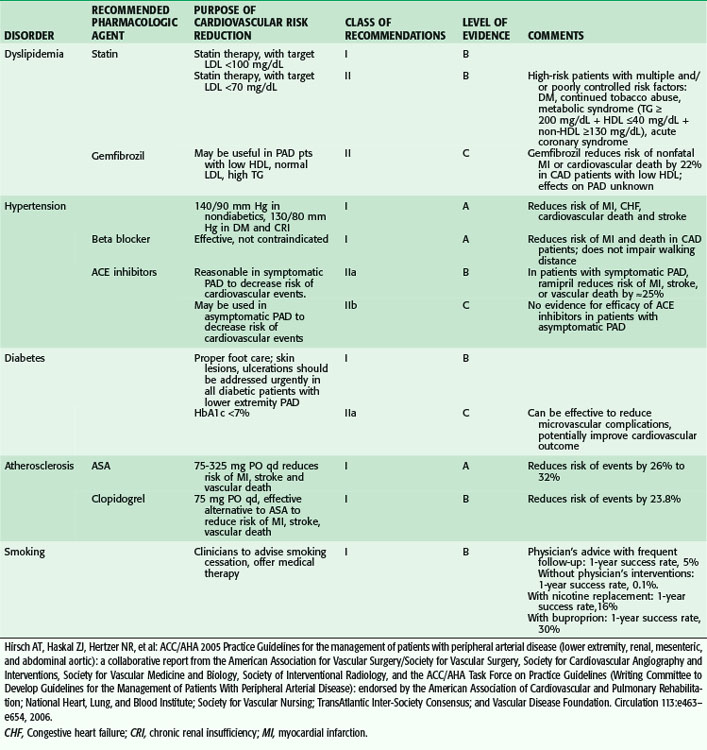
Despite the aging of our population and increasing numbers of people afflicted by atherosclerotic arterial disease, morbidity from myocardial infarction and stroke is decreasing. This is likely secondary to advances in medical management and increasing awareness by affected individuals about the availability of medications that can limit the progression of the disease process. The American Heart Association (AHA) has published guidelines for risk modification that have grown increasingly aggressive in efforts to treat this important public health concern. In contemporary surgical practice, lipid modification, antiplatelet and antihypertensive control, and smoking cessation strategies are all becoming standard management issues for the patient with vascular disease. Table 63-5 summarizes the AHA guidelines for risk factor modification.
Risk factors contributing to PAD are the same as those for atherosclerosis:
Revascularization: Surgical Treatment
Intermittent Claudication
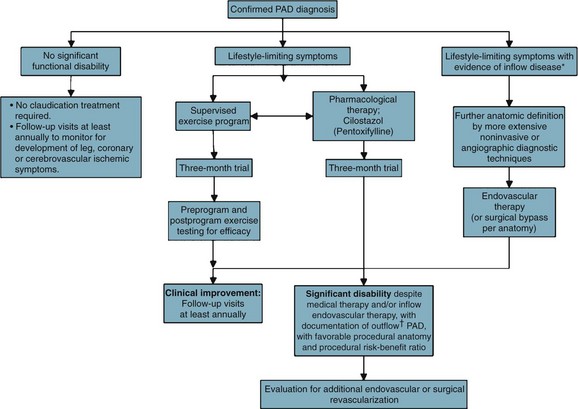
Patients with intermittent claudication (IC) are treated by risk factor modification to decrease their risk of myocardial infarction (MI) and cerebral vascular accident (CVA). A trial of cilostazol and supervised exercise is recommended; these therapies, combined with risk factor modification (particularly smoking cessation), have been shown to improve walking distance. Patients are reassured that they are at limited risk of limb loss, approximately 2% to 3% at 5 years. Although significant disability may occur as a result of IC, symptoms remain stable because of the development of collateral flow, or perhaps alterations in gait that favor nonischemic muscle groups.7 However, 25% of IC patients will see deterioration in their clinical course, usually during the first year after diagnosis; the best predictor of this decline is the initial ABI. Patients with an initial ABI of less than 0.50 have a hazard ratio of more than 2 compared with patients with an ABI higher than 0.50. IC patients with an initial ankle pressure of 40 to 60 mm Hg have an annual limb loss rate of 8.5%.
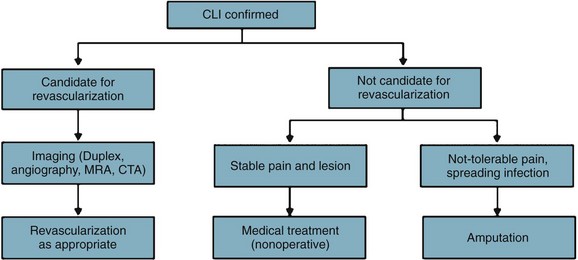
Patients who present initially with low ankle pressures or absent femoral pulses, or patients who return with unabated, severe, lifestyle-limiting symptoms that have not adequately responded to nonoperative measures, are considered for intervention (Fig. 63-10).
Critical Limb Ischemia
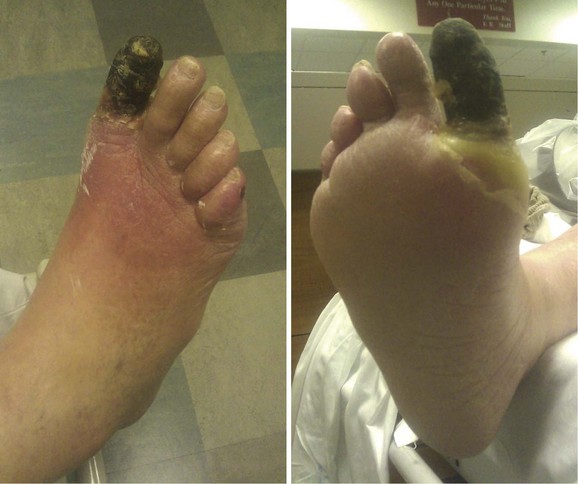
Similarly, patients who present with nonhealing wounds of the feet, dry gangrene, or necrotizing infection are offered an expeditious workup to plan a revascularization that will reestablish in-line blood flow to the foot. In case of tissue loss with infection, an immediate decision regarding the need for operative débridement or amputation prior to revascularization must be made. In case of severe sepsis with hemodynamic instability or evidence of multisystem organ failure, patients may require amputation prior to revascularization. However, if a patient with systemic toxicity from the infection responds rapidly to administration of IV antibiotics, revascularization prior to débridement may minimize tissue loss. See Figure 63-11.
Diabetic Foot
PVD is common among patients with diabetes (Fig. 63-12). IC is twice as common among diabetic patients than among nondiabetic patients. An increase in HgbA1C by 1% can result in more than a 25% risk of PAD. Major amputation rates are five to ten times higher in diabetics than nondiabetics. Because of these causal relations, the American Diabetes Association recommends ABI screening every 5 years in patients with diabetes.8
Lower Extremity Amputations
Amputation, unfortunately, in the minds of most surgeons and their patients, represents a failure of therapy or care. Consent for this operation, regardless of the level, is usually imbued with an emotional gravity that few other, even more complex, dangerous, life-altering procedures carry. Not infrequently, amputations in the vascular patient are prone to breakdown and the need for revision is common, thereby prolonging the patient’s time in the hospital, lengthening the recovery process, decreasing the chances of functional recovery, and contributing to a high rate of depression. It is therefore incumbent on the surgeon to ensure that all steps are taken to minimize the risks of local and systemic complications.9
The perioperative mortality rate for below-knee amputation (BKA ) is 5% to 10% and that of above-knee amputation (AKA) even higher, 10% to 15%, testifying to the limited reserves of patient facing these procedures.10 Wound healing in BKA is poor; almost one third of patients require débridement or healing by secondary intention or conversion to AKA (Fig. 63-13). Despite optimistic preoperative counseling, functional recovery with ambulation is poor for AKA patients.11
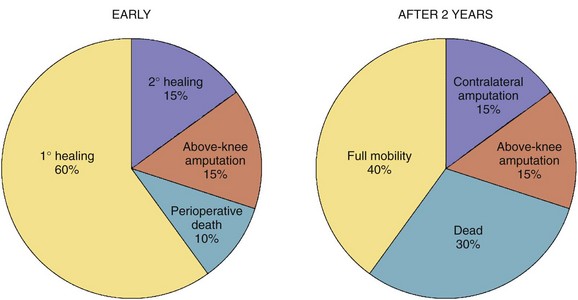
FIGURE 63-13 Early and 2-year outcomes of the BKA patient.
(From Norgren L, Hiatt WR, Dormandy JA, et al: Inter-Society Consensus for the Management of Peripheral Arterial Disease [TASC II]. J Vasc Surg 45[Suppl]:S5–S67, 2007.)
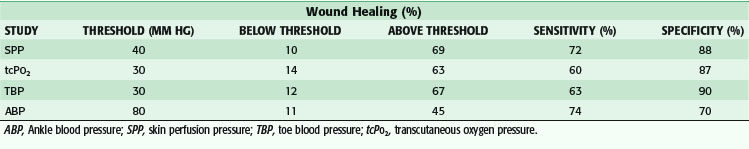
The determination of the appropriate level for amputation has been studied extensively (Table 63-6). In an effort to preserve limb length and decrease the metabolic demands of ambulation, toe and transmetatarsal amputations (TMAs) are usually attempted. Aside from clinical judgment, segmental arterial pressures, Doppler waveforms, and toe pressures have been studied. Diabetes, combined with a toe pressure of lower than 30 mm Hg, has been correlated with failure of healing minor amputations. Transcutaneous O2 pressure (TcPO2) measurement, easily obtained via a small sensor placed on the skin in the area of proposed amputation, has an accuracy of higher than 87% for predicting wound healing. A reading higher than 40 mm Hg is associated with successful healing, whereas TcPO2 less than 20 mm Hg is associated with failure. Absolute ankle pressure higher than 60 mm Hg has been shown to predict the healing of BKAs with an accuracy of 50% to 90%.12
Ray Amputation
For ray amputation, a tennis racquet incision around the base of the affected toe is made. For first toe amputations, the handle of the racquet is oriented along the medial aspect of the metatarsal head; for the fifth toe, it is oriented laterally. For toes 2 to 4, the incision is along the dorsal midline (Fig. 63-14). Neighboring digital vessels are carefully preserved as the soft tissues are divided. The extensor tendons are divided under tension and permitted to retract. The bone is divided proximal to the metatarsal head. If sesamoid bones are encountered, these are removed. Plantar soft tissue is divided; flexor tendons are similarly allowed to retract after being divided under tension. Soft tissue is closed over the metatarsal head with absorbable sutures. Minimal handling of the skin prevents ischemic trauma. The skin is approximated without tension or left open for closure by secondary intention.
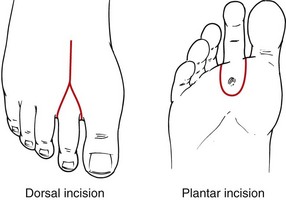
FIGURE 63-14 Surgical approach to ray amputation.
(From Eidt JF, Kalapatapu VR: Techniques and results. In Cronenwett JL, Johnston W [eds]: Rutherford’s vascular surgery, ed 7, Philadelphia, 2010, Saunders, pp 1772–1790.)
Transmetatarsal Amputation
A curvilear incision is made above the metatarsal heads, with an intentionally longer flap fashioned on the plantar surface (Fig. 63-15). Soft tissues anterior to the bone are divided, including the tendons of the extensor muscles. Digital arteries are suture-ligated as needed. A periosteal elevator is applied to elevate the soft tissues just to the point of division. An oscillating saw is used to divide the metatarsals behind their heads. The plantar tendons and plantar soft tissues are divided. The wound is irrigated using a mechanical lavage system and inspected for hemostasis. The soft tissue is reapproximated over the bone using absorbable sutures. The skin is reapproximated, with minimal manipulation and without tension, using interrupted nylon vertical mattress sutures. Non–weight-bearing status is encouraged for at least 4 weeks. In case of infection, a guillotine procedure may be performed and a vacuum dressing applied, with placement of a split-thickness skin graft after the wound bed has adequately granulated.
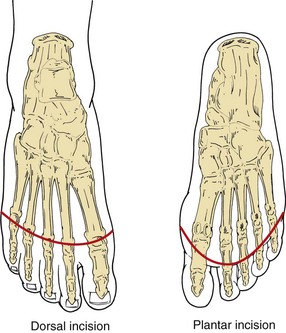
FIGURE 63-15 Surgical approach to transmetatarsal amputation.
(From Eidt JF, Kalapatapu VR: Techniques and results. In Cronenwett JL, Johnston W [eds]: Rutherford’s vascular surgery, ed 7, Philadelphia, 2010, Saunders, pp 1772–1790.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree