PERINATAL CIRCULATORY PHYSIOLOGY
Introduction
Fetal circulation uses the placenta for exchange of respiratory gases, while postnatal circulation uses the lungs for gas exchange. Consequently, the heart and lungs must adjust to these altering requirements after birth. An appreciation of fetal circulation and the adaptation that it undergoes at birth is important for a better understanding of the postnatal changes of circulation in various types of congenital cardiac defects (CHDs). In this review, I will (1) briefly outline fetal circulation; (2) discuss mechanisms that sustain fetal circulation; (3) succinctly review fetal cardiocirculatory regulation; (4) briefly appraise the influence of cardiac defects on fetal circulation; and (5) describe changes that take place immediately after birth. Postnatal circulatory changes are likely to influence the presentation and neonatal course of important CHDs and will be reviewed in the next chapter. Normal circulation in the adult (Figure 1.1) and in the fetus (Figures 1.2–1.4) are shown in Figures 1.1 through 1.4.
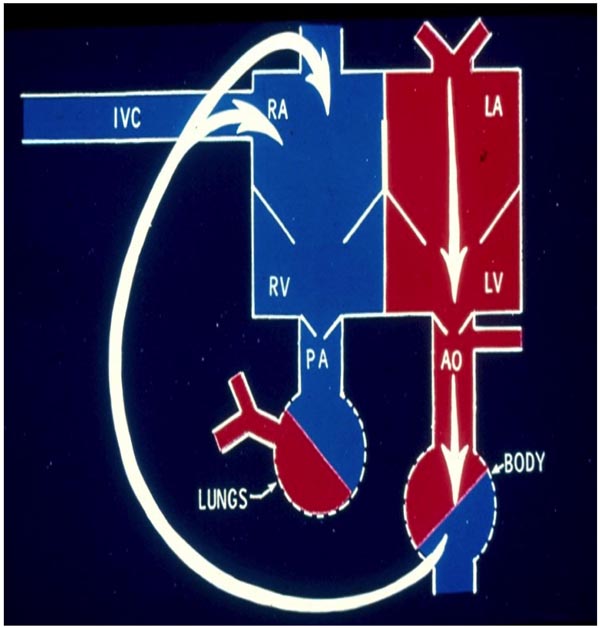
Figure 1.1. Diagrammatic depiction of adult circulation. The systemic venous return comes into the right atrium (RA) via the IVC, SVC (not labeled), and CS (not shown). From there, the blood is transferred to the RV and pumped into the pulmonary artery (PA) and lungs for oxygenation. The pulmonary venous blood returns into the LA and passes on to the LV for pumping into the aorta (AO) and the body. Source: Reproduced with permission from Neonatology Today 2008;3(2):6–12.
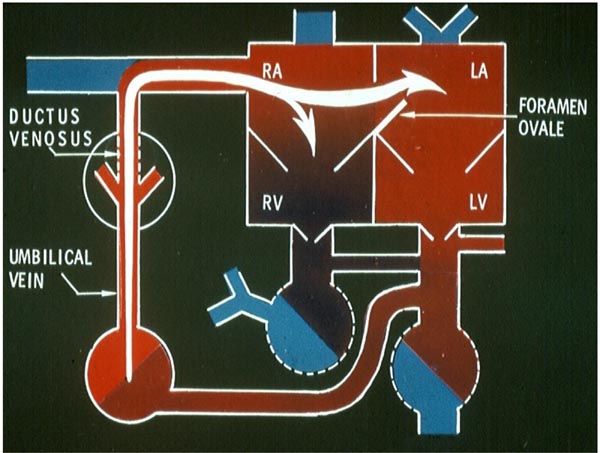
Figure 1.2. Fetal circulatory pathway 1. The venous return from the placenta is transported by the UV and the DV into the IVC (not labeled). From there, a generous proportion is shunted into the LA via the foramen ovale (FO). The residual portion goes into the RV. LV, left ventricle; RA, right atrium. Source: Reproduced with permission from Neonatology Today 2008;3(2):6–12.
Fetal Circulation
Most of the data pertaining to fetal circulation are a result of the investigational interpretation of the data from animal models, particularly the lamb,1–3 although some information from human fetuses is available. The lamb model appears to best represent human physiology.4 Estimates of blood flow are derived by calculations using Fick’s principle, electromagnetic flow measuring devices, utilizing radionuclide-labeled microspheres, and a combination thereof. Cardiac output is expressed as the combined output of both the right and left ventricles under fetal circulatory conditions, while the cardiac output is measured as the volume pumped by each ventricle separately in postnatal circulatory states. More recently, Doppler studies have been used to derive fetal cardiac outputs.5,6
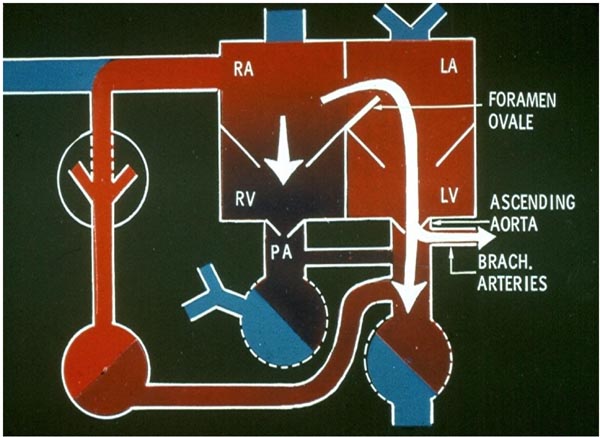
Figure 1.3. Fetal circulatory pathway 2. The blood flowing into the LA via the FO is directed into the LV, and from there pumped into the ascending aorta. Thus, oxygenated blood reaches the brain via the BRACH vessels and the coronary arteries (not shown). The remaining blood goes into the RV. PA, pulmonary artery; RA, right atrium. Source: Reproduced with permission from Neonatology Today 2008;3(2):6–12.
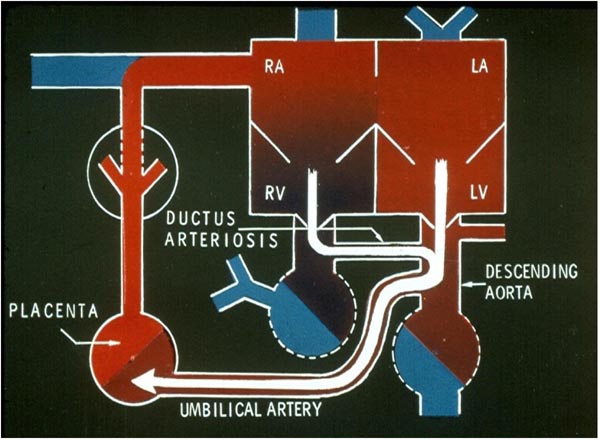
Figure 1.4. Fetal circulatory pathway 3. The right ventricular (RV) blood is propelled into the PA (not labeled). Since the resistance in the pulmonary circuit is high, the majority of the blood is forced via the DA into the descending aorta. From the descending aorta, the desaturated blood goes into the placenta via the umbilical arteries. LA, left atrium; LV, left ventricle; RA, right atrium. Source: Reproduced with permission from Neonatology Today 2008;3(2):6–12.
Course of Fetal Circulation
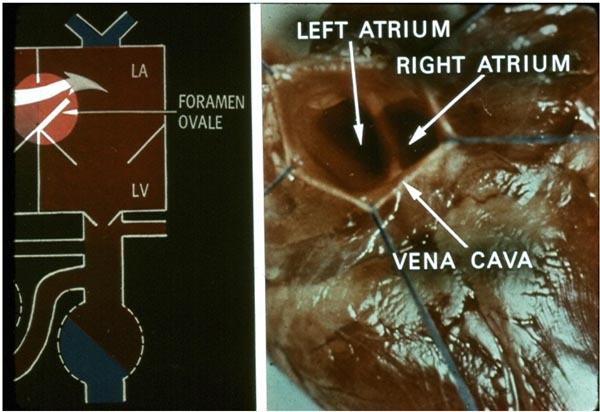
Uptake of oxygen and elimination of carbon dioxide of fetal blood takes place in the placenta. Oxygenated blood from the placenta returns to the fetus via the umbilical vein (UV). At the porta hepatis, the UV is connected to the left and right hepatic veins (HVs) and the ductus venosus (DV). Umbilical venous flow comes into the inferior vena cava (IVC) via the DV (see Figure 1.2). However, some of the umbilical venous blood reaches the IVC via the portal veins, liver, and HVs. Because higher resistance is offered by the portal veins and liver sinusoids, it is likely that more blood flows through the DV. Once in the IVC, a sizeable amount of the blood preferentially flows into the left atrium (LA) via the widely open FO (see Figure 1.2). This preferential shunting seems to be related in part to overriding of the IVC by crista dividens (free margin of the septum secundum) (Figure 1.5).7 The free edge of the septum primum (lower margin of the FO) is slanted to the left side, and the FO is kept open by the IVC blood stream (see Figures 1.2 and 1.5). Furthermore, the Eustachian valve (the valve of the IVC) may redirect the IVC blood stream towards the FO.8 Thus, the oxygenated blood comes into the LA, LV, and then into the ascending aorta (Figure 1.3). Consequently, the brain, via the brachiocephalic (BRACH) vessels, and the heart, via the coronary arteries (CAs), are supplied with oxygenated blood (see Figure 1.3). A small amount of pulmonary venous return (7%–8% of combined ventricular output [CVO]) does mix with the left atrial blood, but does not appreciably desaturate the large amount of highly oxygenated umbilical venous return. The blood return from the coronary sinus (CS) and superior vena cava (SVC), along with the part of the IVC blood that did not enter the LA, goes through the tricuspid valve (TV) into the right ventricle (RV) (see Figure 1.3). This less-oxygenated blood is pumped into the main PA by the RV (see Figure 1.4). Once in the main PA, the majority of the blood goes into the descending aorta via the ductus arteriosus (DA), with only a small amount of blood going into the lungs. Consequently, the desaturated blood via the umbilical arteries (UAs) goes into the placenta for oxygenation (see Figure 1.4).
Figure 1.5. IVC to LA flow. Magnified view of the fetal circulatory pathways (left panel) displaying the passage of inferior vena caval blood into the LA via the FO. When viewed from below, from the IVC (right panel), a greater portion of the LA is seen, elucidating in part the cause for shunting the inferior vena caval blood into the LA. Crista dividens is more towards the RA facilitating inferior vena caval flow into the LA. LV, left ventricle. Source: Reproduced with permission from Neonatology Today 2008;3(2):6–12.
Mechanisms of Maintenance of Fetal Circulation
DV and Umbilical Vessels These structures are believed to be kept patent by the mechanical effect of blood flow through them.
FO The mechanical effect of streaming of the inferior vena caval blood into the LA (Figures 1.2 and 1.5) as well as the temporal relationship of the IVC to the LA (see Figure 1.5) keeps the FO open in the fetus.
DA The muscular nature of the DA dictates that it may have to be kept open by active dilation; the low PO2 to which the DA is exposed and prostaglandins (PGs) are likely to be responsible for patency of the DA. Multiple studies looking at this phenomenon imply that both the locally formed and circulating PGs (E2 and I2) may account for this effect. The PGs are rapidly cleared by passage through the lungs; however, this may not have an adverse influence on the level of PGs since pulmonary blood flow (PBF) is very low in the fetus, approximately 7% to 8% of the CVO. Thus, the circulating PGs remain high in the fetus. This is in contradiction to what happens in postnatal life, where the PGs are rapidly cleared by passage through the lungs. Furthermore, the placenta produces a large amount of PGs. The patency of the DA may also be interrelated to circulating adenosine.9
Relative Flows to the Placenta and Lungs The pressures in the main PA and descending aorta are identical because the interconnecting DA is large. Therefore, the distribution of blood flow to the placenta versus the lungs is largely dependent on the relative resistances offered by them. Because the placenta has a low-resistance circuit, a larger fraction of the blood goes into the placenta. Since the pulmonary circulation is a high-resistance circuit, a smaller percentage of the CVO gets distributed into the lungs.
High Pulmonary Vascular Resistance (PVR) The reasons for elevated PVR are not clearly known. A number of hypotheses have been advanced, and these include kinking and a high level of tortuosity of the small pulmonary blood vessels10 and thick smooth muscle layer of the pulmonary arterioles.11,12 However, there is no general agreement on the causative relationship. High pulmonary arteriolar resistance may be related to the low partial pressure of oxygen to which the pulmonary arterioles are subjected. Low PO2 keeps the arterioles thick and constricted. The PVR may also be controlled by changes in the pH and PCO2 and by the autonomic nervous system.11,12 In addition, many endogenous and exogenous vasoactive substances may also stimulate the fetal pulmonary blood vessels. Apart from the low oxygen tension mentioned above, low production of vasodilators such as nitric oxide (NO) and PGs and high levels of vasoconstrictors such as endothelin-1 and thromboxane may also contribute to the high PVR.
Some studies reveal that pharmacological amounts of PGs have a remarkable effect on fetal pulmonary circulation.11,13 PG F2α and leukotrienes (LTD4) incite pulmonary vasoconstriction, while PGs E1, E2, and I2 produce pulmonary vasodilation.14 The function of PGs in keeping a normally increased PVR has not been clearly defined. It is likely that PGs mediate the hypoxic stimulus.
Distribution of the Cardiac Output and Oxygen Saturations in the Fetus
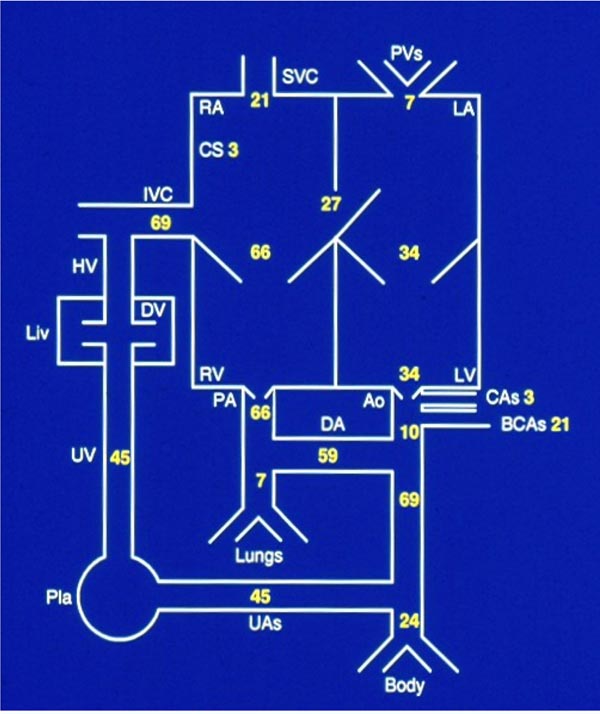
As pointed out in the above sections, cardiac output in the fetus is expressed as CVO. The CVO in the lamb is 450 to 500 ml/kg/minute. The guesstimate of relative distribution of the CVO is based on the information derived from chronically instrumented lambs15,16 and is depicted in Figure 1.6. Notable features are that the RV pumps two-thirds of CVO, but only 7% to 8% of CVO goes into the lungs (lungs do not serve gas exchange function in the fetus), while only 10% of CVO passes through the aortic isthmus, and slightly less than half of CVO traverses the UAs into the placenta for oxygenation. It should be noted that the data may be different in the human fetus in view of the larger brain size in the human, although the reported data have a high degree of variance.17 However, it is most likely that values in the human fetus are qualitatively similar to those seen in the lamb model, but in the human fetus, the RV output is smaller and the percentage of blood received by the brain is higher than that of the lamb.
Figure 1.6. Distribution of cardiac output in the fetus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree