, Ron Blankstein2, Gregory D. Lewis3 and Gregory D. Lewis4
(1)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School Cardiovascular Medicine, Department of Medicine and Radiology, Brigham and Women’s Hospital, Boston, MA, USA
(3)
Harvard Medical School, Boston, USA
(4)
Mass General Cardiopulmonary Exercise Laboratory, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Pericardial diseases and associated hemodynamic findings represent an important but difficult area in cardiovascular physiology and disease. In this chapter, we will discuss several different key aspects of pericardial physiology and pathology, including acute and chronic inflammatory states of the pericardium, cardiac tamponade, and chronic constrictive pericarditis. The outline of each section will include a discussion of the etiology of the specific pericardial pathology, a description of salient diagnostic features (by physical examination, echocardiography, hemodynamic measurements, and/or advanced imaging techniques, where applicable), and a discussion of management and follow-up.
Electronic supplementary material
The online version of this chapter (doi:10.1007/978-1-4471-4483-0_18) contains supplementary material, which is available to authorized users.
Abbreviations
ADA
Adenosine deaminase
ANA
Antinuclear antibody
CAD
Coronary artery disease
COPD
Chronic obstructive pulmonary disease
CRP
C-reactive protein
CT
Computed tomography
EBV
Epstein-Barr virus
ECG
Electrocardiogram
EP
Electrophysiology
ESR
Erythrocyte sedimentation rate
HBV
Hepatitis B virus
HCV
Hepatitis C virus
IPP
Intra-pericardial pressure
IVC
Inferior vena cava
LV
Left ventricular
LVEDP
Left ventricular end diastolic pressure
MRI
Magnetic resonance imaging
NSVT
Nonsustained ventricular tachycardia
PE
Pulmonary embolism
PEEP
Positive end expiratory pressure
RA
Rheumatoid arthritis
RV
Right ventricular
RVEDP
Right ventricular end diastolic pressure
RVSP
Right ventricular systolic pressure
SLE
Systemic lupus erythematosus
TB
Tuberculosis
TEE
Transesophageal echocardiography
TMP-SMX
Trimethoprim–sulfamethoxazole
Introduction
Pericardial diseases and associated hemodynamic findings represent an important but difficult area in cardiovascular physiology and disease. In this chapter, we will discuss several different key aspects of pericardial physiology and pathology, including acute and chronic inflammatory states of the pericardium, cardiac tamponade, and chronic constrictive pericarditis. The outline of each section will include a discussion of the etiology of the specific pericardial pathology, a description of salient diagnostic features (by physical examination, echocardiography, hemodynamic measurements, and/or advanced imaging techniques, where applicable), and a discussion of management and follow-up.
Normal Physiology of the Pericardium
Anatomy:
Two layers: the outermost fibrous pericardium and the inner 2-component serous pericardium (visceral and parietal); approximately 2 mm thick in non-pathologic conditions
Pericardial sac between the visceral and parietal contains 35–50 ml of serous fluid
Pericardium is by pericardial reflections, with the left atrium and pulmonary veins mostly outside the pericardium
Phrenic nerves run adjacent to the parietal pericardium (accounting for referred phrenic nerve irritation during pericardial inflammation)
Pericardial arteries supply blood to the dorsal portion of the pericardium
Physiology: Normal pericardium serves several key roles:
“Pericardial restraint”: the compliance of the pericardium varies with the volume in the heart; initially, the pericardium is supple, and can expand with minimal increases in intrapericardial pressure with cardiac filling; at higher volumes, the intrapericardial pressure rises, and impedes systemic and pulmonary venous return (Fig. 18-1). This explains why acute increases in pericardial fluid volume lead to very high intrapericardial pressures and impedance to cardiac filling and ejection (e.g., cardiac tamponade) whereas slowly growing effusions are more well tolerated
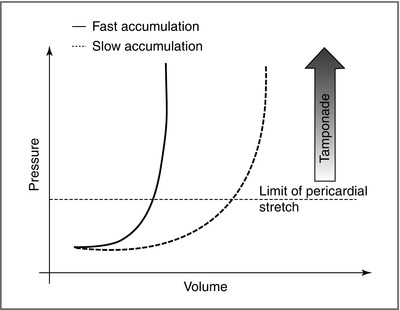
Figure 18-1
Pericardial restraint. Slow accumulation of pericardial fluid leads to a “give” in the pericardium, gradual increase in pericardial compliance, and a less exuberant rise in intrapericardial pressure (a higher threshold for tamponade). In rapid accumulation, the gradual increased pericardial compliance is not permitted, and tamponade ensues at a lower pericardial fluid volume (Courtesy of Dr. Hanna Gaggin)
Barrier to infection
Respiratory-cardiac coupling (pericardium transmits intrathoracic pressure to the heart; for example, during inspiration, intrapleural pressure falls; normal pericardial function will transmit a negative intrapleural pressure to the heart, to decrease intracardiac pressures and provide an impetus for systemic venous return)
Acute Pericarditis
Acute pericarditis is an acute inflammatory process of the pericardium affecting both layers of the pericardium. Here, we review the clinical profile, evaluation, management, and follow-up of patients with acute pericarditis
Etiology (Table 18-1) – etiology often not known and ∼80 % are considered idiopathic [1]
Table 18-1
Etiologies and therapies of acute pericarditis
Etiology
Evaluation
Treatment
Idiopathic
Standard
NSAIDs
Infectious (e.g., viral, bacterial, fungal, TB)
Viral titers/cultures, HIV, pericardial fluid analysis (ADA and PCR for TB), pericardial biopsy (rare)
Anti-infective therapy, drainage of fluid (for bacterial)
Post-MI
Standard
Aspirin (no NSAIDs due to infarct remodeling), avoid anticoagulation if possible
Aortic dissection
Urgent echocardiography or cardiac CT/MRI
Surgical (do not drain percutaneously)
Traumatic
Urgent echocardiography or cardiac CT/MRI
NSAIDs, avoid anticoagulation, close monitoring
Cancer
Standard, pericardial fluid cytology or flow cytometry/tumor markers
NSAIDs or intra-pericardial steroids, pericardial window for refractory cases
Renal failure
Standard
Intensify dialysis
Autoimmune disease
ANA, ESR, CRP, complement, anti-dsDNA
Rheumatologic evaluation, NSAIDs or steroid therapy (anti-inflammatory therapy)
Post-cardiotomy
Standard
NSAIDs, avoid anticoagulation
Drug reaction
Can be linked to autoimmune markers (see above)
Discontinue offending agents; NSAID or steroid therapy
Infections in immunocompetent hosts
Viral (e.g., HIV, coxsackievirus, adenovirus, parvovirus B19, HCV/HBV)
Bacterial (e.g., via extension from lung infection, endocarditis; Lyme disease, tuberculosis)
Infections in immunocompromised hosts
Same as above, plus a variety of fungal or mycobacterial infections
Autoimmune (e.g., SLE/RA, dermatomyositis, vasculitis)
Post-myocardial infarction (Dressler) syndrome (weeks after large, transmural infarction; associated with markers of systemic inflammation, e.g., elevated ESR, fever)
Drugs (e.g., procainamide, hydralazine)
Cancer (e.g., via direct anatomic extension, pericardial metastases, or cardiac invasion; e.g., EBV-associated non-Hodgkin’s lymphoma, melanoma, sarcoma, pulmonary)
Radiation (acutely after radiation)
Post-pericardiotomy syndrome (e.g., after cardiac surgery; usually more prominent effusions present after aortic or mitral valve surgery)
Hemopericardium (e.g., from aortic dissection into the pericardium, traumatic, iatrogenic – EP ablation procedures, or anticoagulation-associated spontaneous bleed)
Endocrine disorders (e.g., thyroid disorder)
Uremia (e.g., patients with stage V chronic kidney disease)
Clinical presentation—History and Physical Examination:
Acute onset chest pain (sharp, pleuritic, radiation to the ipsilateral shoulder, neck and trapezius, better with sitting forward, worse while lying back)
Can be associated with subjective dyspnea, palpitations/supraventricular arrhythmias (e.g., new onset atrial fibrillation), or hiccups (e.g., phrenic nerve irritation)
Nearly 85 % have a pericardial friction rub on auscultation heard best at left lower sternal border; rub = movement of pericardial surfaces against each other during cardiac motion (up to 3 components: 1—ventricular systole; 2—early ventricular filling; 3—atrial contraction); variable during acute pericarditis [2]
Assessment should include signs of heart failure or ventricular irritability (e.g., NSVT; indicating concurrent myocarditis) or tamponade (see below)
History should focus on etiology of pericarditis (e.g., concurrent malignancy, rheumatologic disorders, recent viral infections, etc.)
Clinical presentation—Electrocardiography:
Classically described as diffuse ST segment elevation with reciprocal PR depression (most prominent in leads II and V5–V6; Fig. 18-2). However, opposite pattern in lead aVR where PR segment elevation may be seen and is a specific marker for pericarditis
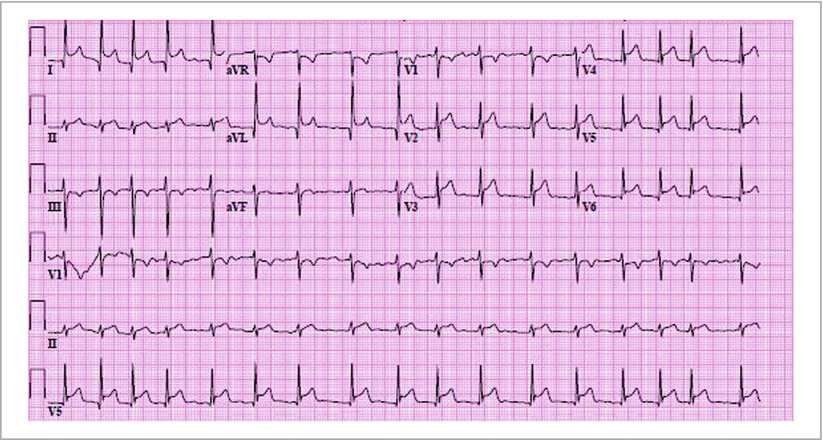
Figure 18-2
12-lead electrocardiogram of an 80 year old male with an aortic dissection complicated by severe aortic insufficiency and rupture of aorta into pericardium, resulting in acute pericarditis. Note rapid atrial fibrillation with associated PR depression and diffuse ST elevation
Note: ECG changes can be localized to one territory, and PR changes can occur independent of ST elevations
Acute pericarditis can be differentiated from acute MI by (1) absence of reciprocal ST depression, (2) convexity of ST segment (vs. concave down with MI), (3) rapid evolution of ST segments to biphasic T waves and T wave inversion; (4) appearance of Q waves and (5) loss of R wave height
Acute pericarditis can be differentiated from normal variant repolarization by ST to T wave height ratio in lead V6 > 0.25 [3]
ECG in acute pericarditis can progress through 4 phases: (1) ST segment elevation; (2) normal; (3) T wave inversions in same leads; (4) normal. These changes occur over a variable time period (e.g., weeks to months); nearly 80 % patients with acute pericarditis have ST-elevations at the time of initial presentation [4]
Clinical presentation—Laboratory and imaging findings
Non-specific
Elevation in ESR or CRP
Troponin elevated in nearly 50 % of patients in one series with pericarditis, specifically in those with ST elevation [5]; no real prognostic importance [6]
ANA often positive (∼40 %) although titer usually 1:40–1:80. If higher → suspect rheumatologic disease [7]
Chest x-ray, echocardiography usually unremarkable; used to exclude pulmonary disease, heart failure, and/or cardiac tamponade
Cardiac MRI: pericardial inflammation/increased T2 signal of pericardium (Fig. 18-3a, b). MRI can differentiate myopericarditis (sub-epicardial enhancement) from infarction (sub-endocardial enhancement) with high accuracy.
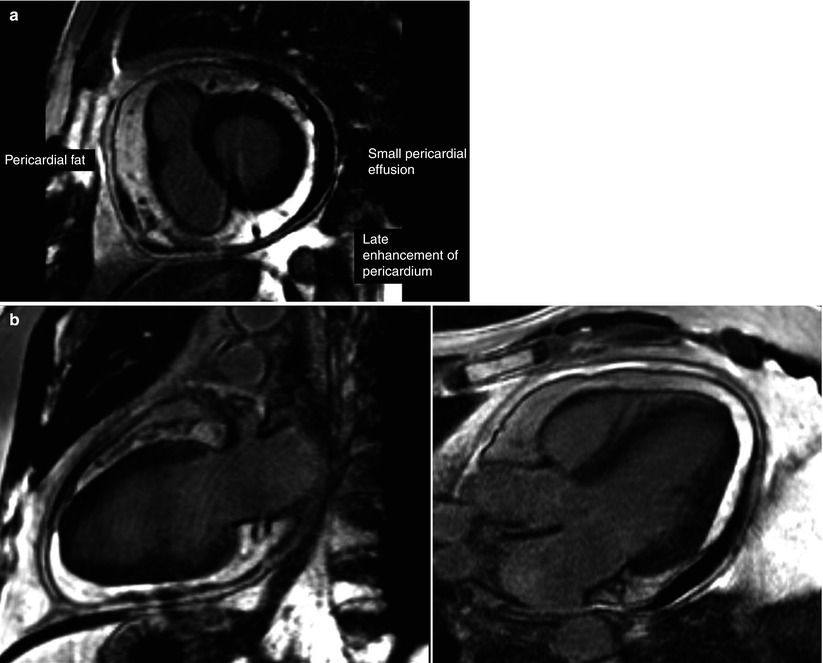
Figure 18-3
Late gadolinium enhancement imaging, indicating enhancement of the visceral and parietal pericardium and an associated pericardial effusion in a short-axis view (a), two-chamber view (b-left), and three-chamber view (b-right)
Acute pericarditis: Clinical evaluation & examination
Standard evaluation should include complete blood count (rule-out infection), renal function (rule-out uremia), chest x-ray (concomitant disease), echocardiography (underlying ventricular disease, effusion), cardiac troponin (serially, to exclude significant myocardial injury/acute MI), thyroid stimulating hormone level, HIV testing, age-appropriate cancer screening and ESR (to assess for disease activity)
Additional testing can be conducted on the basis of focused physical examination and history, including viral panels, blood investigations for infectious causes, serum rheumatologic studies (e.g., ANA panel), additional imaging studies to search for malignancy
Cardiac MRI (for assessment of pericardial enhancement) may be used to confirm diagnosis; identify/exclude concomitant myocarditis or myocardial infarction. Coronary angiography may be considered in cases where clinical presentation is unclear with regard to acute MI vs. pericarditis, particularly if MRI not available and high suspicion for obstructive CAD
Coronary CT angiography may be useful for excluding obstructive CAD in selected cases when suspicion for obstructive CAD lower; pericardial contrast enhancement may be visualized on CT to support presence of pericardial inflammation.
Acute pericarditis: Management (idiopathic only)
Limited randomized evidence in the management of acute pericarditis
Does the patient have to be hospitalized? Indicators of adverse clinical profile [8, 9]:
Fever
Subacute onset of symptoms (e.g., weeks-months; indicating more insidious underlying disorder)
Failure of aspirin or NSAID therapy (after at least 7 days)
Trauma
Oral anticoagulation
Evidence of myocarditis
Large effusion or tamponade
It is important to recognize that these recommendations are for patients with idiopathic (or viral) pericarditis only; pericarditis associated with other specific underlying diseases (e.g., malignancy, trauma, aortic dissection) are treated differently
First-line therapy: non-steroidal anti-inflammatory agents (e.g., ibuprofen 800 mg TID, indomethacin 80 mg TID) or high dose aspirin (650 mg QID) in patients with pericarditis associated with MI (as NSAIDs can impair infarct healing)
Usual duration: 14 days
Relieve chest pain in up to 90 %, generally within days of initiation [4]
Adjunct colchicine has been advocated as an initial adjunctive therapy with NSAIDs to prevent recurrent pericarditis (has been studied in small trials; dose 1–2 mg loading, 0.5–1.0 mg daily for 3 months) [10]
Second-line therapy: If initial therapy is unsuccessful at relief of chest pain, add colchicine therapy and/or switch to a different NSAID [4]
Third-line therapy: If these efforts fail, initiation of steroid therapy (prednisone 1–1.5 mg/kg/day) may prove useful, with very slow taper over the course of months
If higher dose steroids are employed, concomitant clinical prophylaxis should be implemented (e.g., TMP-SMX for P. jiroveci pneumonia; proton pump inhibitors, and osteroporosis protection)
Steroids may increase risk of recurrent pericarditis and constriction
Reserved for refractory pericarditis
Acute pericarditis—Follow-up:
Patients should be advised to refrain from vigorous exercise given the risk of ventricular and supraventricular arrhythmias until completion of NSAID therapy and resolution of symptoms
Follow-up echocardiography is warranted if initial echocardiography demonstrated effusion (of any size) or other abnormalities; note that “constrictive physiology” by echo (see below) during initial evaluation of pericarditis should be re-assessed after a period of treatment (e.g., acute pericardial inflammation can mimic chronic constrictive pericarditis; the diagnosis of constriction should be made after recovery of acute pericardial inflammation, if possible)
Up to 30 % patients with acute pericarditis will have recurrent pericarditis [4]
Cardiac Tamponade
Major issues involving pericardial effusions revolve around (1) assessment of clinical significance (e.g., progression to tamponade) by clinical, imaging, and hemodynamic parameters and (2) therapy. Given the overlap in etiology between acute pericarditis and pericardial effusions, we will focus here on clinical evaluation and therapy of cardiac tamponade, with specific emphasis on physical examination, imaging, and hemodynamics
Cardiac tamponade—hemodynamics:
Cardiac tamponade refers to impedance to cardiac filling secondary to increased intrapericardial pressures (IPP): accumulation of fluid in the pericardial sac increases IPP; when IPP exceeds central venous pressure (CVP), systemic venous return (preload) is reduced, and right ventricular (and left ventricular) stroke volume falls by a Frank-Starling mechanism. Although compensatory tachycardia may assist in initially maintaining cardiac output, hemodynamic collapse, hypotension, and cardiogenic shock will eventually occur< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


