Pericardial Diseases
Jonathan N. Johnson
Frank Cetta
Anatomy and Physiology
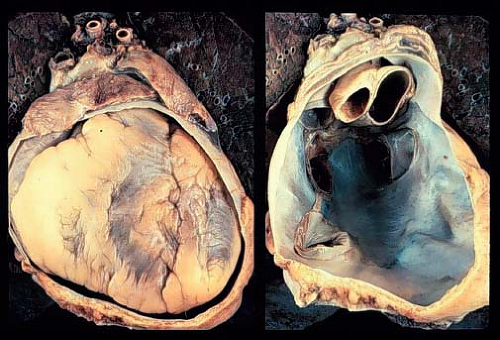
The pericardium is comprised of two principal layers, the visceral pericardium and the parietal pericardium. The visceral pericardium, or epicardium, is a single serous layer covering the surface of the heart and proximal great vessels (Fig. 61.1). The parietal pericardium consists of three layers. The innermost layer is a serous layer, continuous with the serous visceral pericardium. The space between the visceral serous and parietal serous layers is the pericardial space, and contains a small amount of serous fluid for lubrication (<20 to 30 cc in adults, less in children). The middle layer of the parietal pericardium is fibrous, while the outer layer is epicardial collagenous connective tissue. The pericardium receives arterial blood supply from the descending aorta and internal mammary artery, and is innervated from the phrenic and vagus nerves. Within the thoracic cavity, it is anteriorly bordered by the sternum, inferiorly by the diaphragm and a portion of the inferior vena cava, and posteriorly by the esophagus, aorta, pulmonary veins, and thoracic vertebrae (1,2).
The pericardium provides mechanical protection to the heart from the spread of neoplastic, infectious, and inflammatory diseases from adjacent structures. The presence of a small amount of fluid in the pericardial space allows for free movement of the heart throughout the cardiac cycle. The pericardium limits acute distension of the heart, and therefore limits end-diastolic volume. It permits diastolic coupling of the two ventricles, whereby filling pressure abnormalities of one ventricle affect the other. Slow progressive accumulation of fluid within the pericardium is tolerated by stretching and growth of the parietal pericardium, however, rapid accumulation of even a small amount of fluid is poorly tolerated (3).
Acute Pericarditis
Symptoms
Acute pericarditis may present with precordial or substernal chest pain. The pain is described as squeezing, sharp or dull, and characteristically is worse in the supine position. The patient will prefer to sit upright leaning forward, and may refuse to lie down to be examined. The pain worsens with inspiration, coughing, and movement (4,5). Younger children may present with atypical symptoms. Respiratory distress is uncommon unless tamponade or pulmonary disease is present. Rarely, abdominal pain can result from hepatic distension in patients with quickly accumulating effusions. Fever may be present.
Physical Examination
The pathognomonic physical finding in patients with acute pericarditis is a friction rub. This is a high-frequency, scratching or sandpaper-like sound caused by friction between the inflamed pericardial surfaces. The rub can be heard throughout the cardiac cycle; however, it may be intermittent. It often is heard best at the left sternal border or the apex. The rub is loudest when the heart is closest to the chest wall, such as when the patient leans forward, kneels, and/or inspires (6,7). Absence of a friction rub does not exclude pericarditis, particularly in patients with large effusions. In patients with large effusions or tamponade, the heart sounds may be muffled.
Cardiac Tamponade
Cardiac tamponade occurs when the heart is compressed by a fluid-filled pericardium. This causes restriction of ventricular and atrial filling and decreased cardiac output (8). Tamponade results from a sudden increase in pericardial fluid volume or from progressive increase in volume beyond the point of potential pericardial distention. On examination, tamponade is characterized by Beck’s triad: (1) distant heart sounds, (2) hypotension, and (3) elevated central venous pressure with jugular venous distension (9). Patients will have tachycardia, tachypnea, and a narrow pulse pressure with pulsus paradoxus. During the initial stages of tamponade, cardiac output is preserved by increased ejection fraction and heart rate. As these physiologic mechanisms are unable to maintain cardiac output, the patient will become unstable as systemic vascular resistance increases to maintain systemic blood pressure. This will cause the pulse pressure to narrow, with compromised systemic perfusion. Ultimately, decreased coronary perfusion pressure will result in decreased myocardial function, cardiac output, and blood pressure (8,9).
Pulsus paradoxus is defined as a decrease in systolic blood pressure of greater than 10 mm Hg during inspiration. Normally during inspiration, systolic blood pressure decreases by 4 to 6 mm Hg due to decreased intrathoracic pressure and increased capacity of the pulmonary venous bed. With cardiac tamponade, the left ventricular diastolic volume is restricted by increased pericardial pressure, decreased pulmonary venous return, and shifting of the interventricular septum. Clinically, to determine the presence of pulsus paradoxus, the patient should be supine. A blood pressure cuff is inflated until the radial pulse is not palpable. With slow release of pressure, one should listen for the initial Korotkoff sounds. With inspiration, the Korotkoff sounds disappear, particularly in the presence of pulsus paradoxus. Cuff pressure should be slowly released until the Korotkoff sounds are heard throughout the respiratory cycle. The difference in pressure between when the first Korotkoff sound appears and when it is heard with each heart beat is the pulsus. If the pressure difference exceeds 10 mm Hg, pulsus paradoxus is present (10).
During inspiration in normal patients, intrathoracic pressure decreases with an increase in venous return to the right atrium. In patients with cardiac tamponade, however, expansion of the right atrium is limited by pericardial pressure. Thus, during inspiration there may be a paradoxical increase in central venous pressure. This is evident by Kussmaul’s sign; a rise in observed jugular venous pressure during inspiration (3).
Chest Radiography
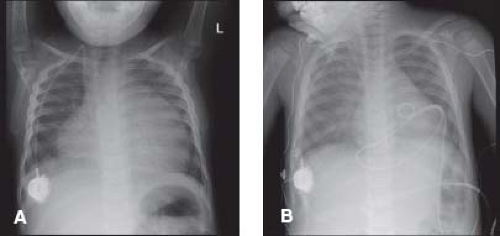
The absence of cardiomegaly by chest radiography does not exclude pericarditis or pericardial effusion. Tamponade can occur in patients with a normal appearing cardiac silhouette. With progressively increasing effusion, the cardiac silhouette may assume a triangular or “water-bottle” shape, with normal pulmonary vascular markings (Fig. 61.2). Patients with chronic pericarditis may have calcification of the pericardium (Fig. 61.3). The remainder of the chest radiograph may suggest potential causes of the pericarditis, including tuberculosis, pneumonia, or neoplastic disease (4,5).
Electrocardiography
The electrocardiographic changes in patients with pericarditis are secondary to direct inflammation of the epi/myocardium or pressure exerted against the epicardium by pericardial fluid. Acute pericarditis is the most frequent cause of ST segment elevation in children. Low QRS voltages occur in all leads in the presence of large pericardial effusions or chronic pericarditis. Electrical alternans, a cyclical variation of the QRS amplitude, may occur secondary to the pendular motion of the heart with a large pericardial effusion.
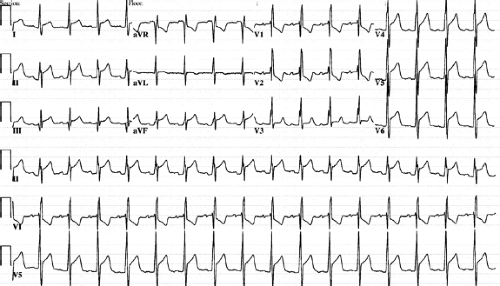
Four stages of electrocardiographic changes have been reported in patients with pericarditis (11). Stage 1 consists of ST segment
elevation in the lateral/inferior leads (I, II, aVF, aVL, V4–V6) (Fig. 61.4). Reciprocal ST segment depression may be present in leads aVR and V1. PR interval depression can occur if atrial tissue is inflamed. In stage 2, the ST segment normalizes, and T-wave amplitude diminishes. In stage 3, the ST segment remains normal, but the T waves become inverted in the lateral/inferior leads (aVF, aVL, V4–V6). Stage 4 is characterized by the relative normalization of the ECG, although some T-wave changes may persist (5,12).
elevation in the lateral/inferior leads (I, II, aVF, aVL, V4–V6) (Fig. 61.4). Reciprocal ST segment depression may be present in leads aVR and V1. PR interval depression can occur if atrial tissue is inflamed. In stage 2, the ST segment normalizes, and T-wave amplitude diminishes. In stage 3, the ST segment remains normal, but the T waves become inverted in the lateral/inferior leads (aVF, aVL, V4–V6). Stage 4 is characterized by the relative normalization of the ECG, although some T-wave changes may persist (5,12).
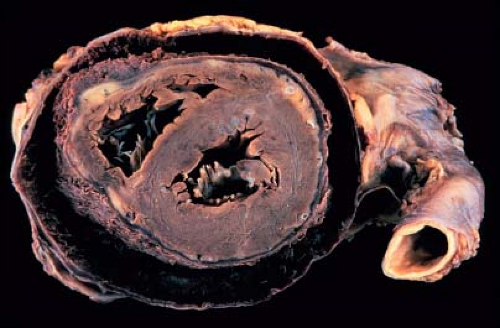
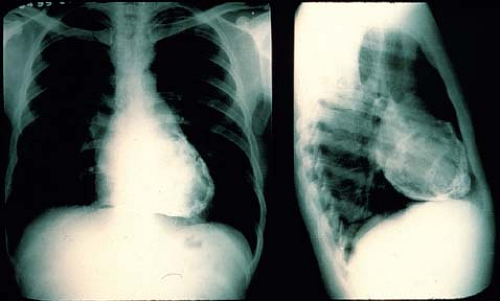 Figure 61.3 Chest radiograph of an adult with chronic pericarditis and resultant calcification of the pericardial space. (Courtesy of Dr. William D. Edwards, Mayo Clinic, Rochester, MN.) |
Echocardiography
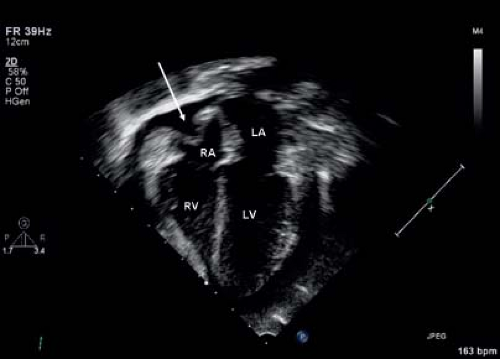
Echocardiography is the primary imaging methodology for the diagnosis of pericardial effusion. Pericardial effusions appear as echo-free spaces around the heart (13). Fibrinous strands (Fig. 61.5), thrombi, adhesions, or metastases may be noted in the effusions. Echocardiography also is helpful in detecting other structural and myocardial causes of cardiomegaly (14).
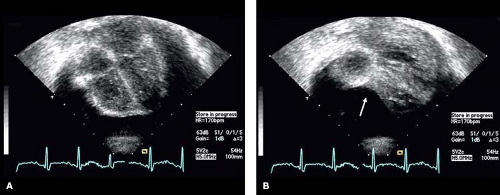
With the patient in the supine position, a small effusion most commonly is seen posteriorly, and may be detectable only in systole. A tiny effusion in systole that is not evident in diastole is normal. An effusion that is present during both systole and diastole is abnormal (15). As the volume of the effusion increases, fluid may be detected both anterior and posterior to the heart (Fig. 61.6). With large effusions, the heart may swing to-and-fro within the pericardial space (Videos 61.1 and 61.2). This swinging movement can be seen by M-mode echocardiography (Fig. 61.7). The earliest
sign of hemodynamic impairment in cardiac tamponade is collapse of the right ventricular free wall in early to mid-diastole (Fig. 61.8) (16). The right atrial free wall may appear indented later in diastole (Fig. 61.9 and Video 61.3). Inferior vena cava dilation without normal inspiratory variation and abnormal ventricular septal motion also may occur. Thrombus in the pericardial space suggests the presence of a hemopericardium.
sign of hemodynamic impairment in cardiac tamponade is collapse of the right ventricular free wall in early to mid-diastole (Fig. 61.8) (16). The right atrial free wall may appear indented later in diastole (Fig. 61.9 and Video 61.3). Inferior vena cava dilation without normal inspiratory variation and abnormal ventricular septal motion also may occur. Thrombus in the pericardial space suggests the presence of a hemopericardium.
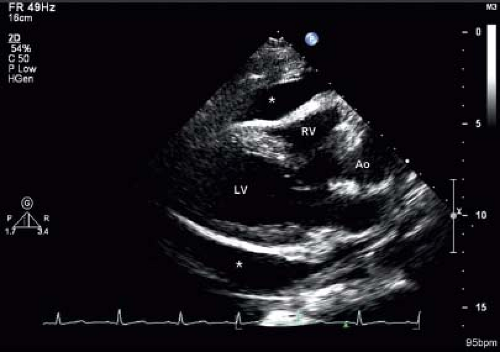 Figure 61.6 Parasternal long-axis echo image showing a large pericardial effusion (asterisk). LV, left ventricle; RV, right ventricle; Ao, aorta. |
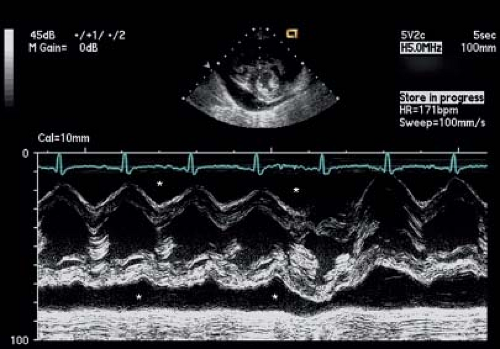 Figure 61.7 M-mode image showing a large pericardial effusion (asterisk) anterior and posterior to the heart. Note the swinging motion of the heart evident in the M-mode signal. |
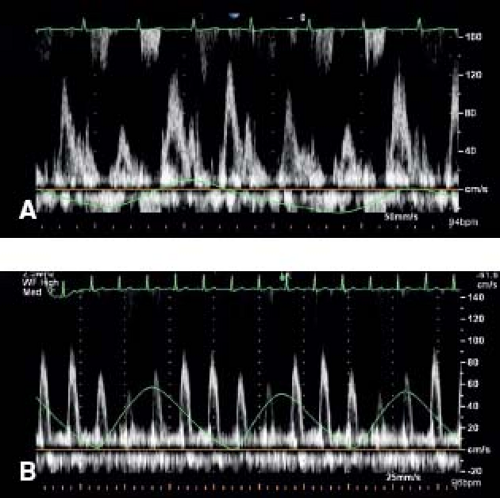
During normal inspiration the intrapericardial and intrathoracic pressures decrease equally. Thus, the left atrial and left ventricular diastolic pressures and the pulmonary capillary wedge pressure decrease equally during inspiration. However in tamponade, during inspiration, the intrathoracic pressure declines to a greater degree than the intrapericardial pressure. Thus, the gradient between the pulmonary capillary wedge pressure and left ventricular diastolic pressures decreases with inspiration. Therefore in cardiac tamponade there is an exaggerated decrease in the mitral inflow velocity (E velocity) and velocity-time integral by at least 30%, with a relatively increased atrial component during inspiration (A velocity) (Fig. 61.10A). Conversely, there is an exaggerated increase in tricuspid inflow velocity (tricuspid E velocity) and the velocity-time integral by at least 70% during inspiration (17,18). The aortic and pulmonary outflow changes mirror those of their respective atrioventricular valves (Fig. 61.10B).
Cardiac Catheterization
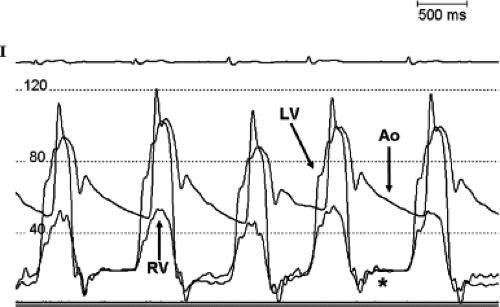
With large accumulation of pericardial fluid, diastolic pressures rise in all four chambers, and ultimately equalize (19). Right ventricular and pulmonary artery pressures may be elevated. Pulsus paradoxus can be seen on a femoral artery pressure tracing (10). In patients with constrictive physiology, due to the equalization of the left ventricular and right ventricular end-diastolic pressures, the characteristic “square root” sign may be present on the left ventricular pressure tracing (Fig. 61.11).
Other Imaging Modalities
MRI or CT can be useful if constrictive pericarditis is suspected. Active pericardial inflammation (a treatable cause of constriction) and hemodynamic evidence of constriction may be apparent using MRI (Fig. 61.12). MRI is helpful in characterizing pericardial masses and congenital anomalies of the pericardium, such as absence of the pericardium and pericardial cysts (20).
Management of Tamponade
After diagnosing cardiac tamponade, one should administer IV fluid immediately to increase diastolic filling pressure temporarily in order to stabilize the patient (21). Medications that decrease systemic arterial blood pressure such as vasodilators and diuretics should be avoided. Indications for pericardiocentesis include low cardiac output, hypotension, pulsus paradoxus >10 mm Hg, suspected bacterial pericarditis, pericardial effusions in immunocompromised hosts, or for diagnostic purposes when the etiology is unclear (22,23).
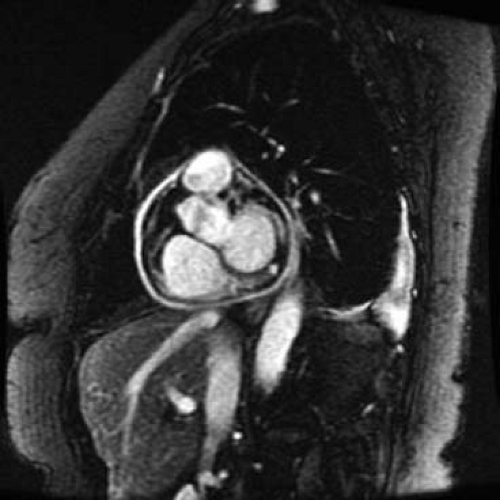 Figure 61.12 Cardiac MRI sequences in a young adult with pericarditis after radiofrequency ablation. Note the extensive pericardial enhancement suggestive of ongoing inflammation. |
Pericardiocentesis should be performed in a monitored setting. The patient should be placed in a 30-degree head-up position and adequately sedated. Continuous monitoring of heart rate, blood pressure, and pulse oximetry should be performed. In an emergent situation, the needle is introduced subxiphoid, and is advanced toward the left shoulder. In nonemergent situations, echocardiographic guidance allows accuracy in entering the pericardial space and reduces complications (23). Echocardiography can be particularly useful in the presence of loculated effusions, and can allow one to place catheters from different access points (parasternal or apical, often wherever the largest amount of pericardial fluid can be visualized). One can perform an agitated saline injection to confirm the location of the needle in the pericardial space. Repeat echocardiography can monitor the adequate drainage of the pericardial fluid and the resolution of tamponade physiology. In the majority of patients, a drainage catheter should be placed (using the Seldinger technique over a wire) for at least 48 hours to detect and drain recurrent effusions (22). Potential complications of pericardiocentesis include death, hemopericardium, pneumothorax, arrhythmias, myocardial puncture, coronary artery, aorta or internal mammary artery injury (23,24).
Pericardial fluid should be analyzed for cell content, glucose concentration, protein concentrations, Gram stain, acid-fast bacilli stain, cultures (bacterial, viral, and fungal), and microscopic analysis (25). Specific bacterial- or viral-specific antigens may be assessed using polymerase chain reaction (PCR) studies and latex agglutination studies. High triglyceride levels are diagnostic of chylopericardium. Adenosine deaminase activity levels can be measured to assist in the diagnosis of tuberculous pericarditis. Increased levels of adenosine deaminase (>40 U/L) accurately diagnose tuberculous pleural effusions (26).
Etiology
Viral Pericarditis
The most common etiology of pericarditis in the pediatric population is viral. The most common viral causes of pericarditis are listed in Table 61.1. Coxsackievirus is the most common viral cause of pericarditis in children (30). Patients often present 10 to 14 days after an upper respiratory or gastrointestinal infection with precordial chest pain, fever, and a friction rub. Some patients, particularly younger children, may present with abdominal pain. Patients with viral pericarditis generally are less-toxic appearing than those patients with
bacterial pericarditis. However, patients with viral pericarditis may appear toxic when there is associated myocarditis. Cardiac tamponade is rare in patients with viral myocarditis; however, patients should be monitored closely for this after initial presentation.
bacterial pericarditis. However, patients with viral pericarditis may appear toxic when there is associated myocarditis. Cardiac tamponade is rare in patients with viral myocarditis; however, patients should be monitored closely for this after initial presentation.
TABLE 61.1 Pericarditis: Potential Viral Causes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
If collected, pericardial fluid usually is serous or serosanguinous, and displays lymphocyte predominance, although neutrophils may be common in the early stages of the illness. Viral cultures can be obtained from the pericardial fluid, nasopharynx, or stool. PCR studies often are useful in determining a specific viral cause (31,32).
Specific treatment for viral pericarditis is symptomatic, including bed rest and oral nonsteroidal anti-inflammatory drugs (NSAIDs). If NSAIDs are unsuccessful, steroids may be considered once bacterial causes have been excluded. The use of colchicine with aspirin as first-line combination therapy decreases the likelihood of recurrence in adults. Colchicine has not been well studied in the pediatric population, but has good anecdotal success and is used in many centers (33). Clinical improvement occurs in days to weeks, with complete resolution usually within 6 weeks. Relapse occurs in a small subset of patients, typically improving with reinstitution of NSAIDs or steroids. Constrictive pericarditis rarely occurs as a late complication of viral pericarditis.
Bacterial Pericarditis
Bacterial pericarditis is a serious, life-threatening disease. It presents most frequently in patients under 2 years of age (34). Patients present with symptoms of fever, chest pain, dyspnea, friction rub, and muffled heart sounds. They also may be very ill and hypotensive on presentation. In addition, patients may have tachycardia out of proportion to the fever. Bacterial pericarditis can result from hematogenous dissemination or direct contact. The lung is the most common origin of dissemination, particularly when the agent is Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae. Septic arthritis, osteomyelitis, meningitis, or soft tissue infection may be sources for hematogenous dissemination (34,35,36,37).
In bacterial pericarditis, the pericardial fluid demonstrates a marked predominance of neutrophils, and cultures typically are positive for the causative organism. Latex agglutination studies of the pericardial fluid, serum, or urine may be helpful if antibiotics have been given prior to obtaining a sample of pericardial fluid. Potential bacterial causes of pericarditis are listed in Table 61.2. Staphylococcus aureus is the most common bacterium isolated, accounting for half of the cases of bacterial pericarditis (34). Also, it is the most common cause of postoperative bacterial pericarditis (i.e., within 3 months of a cardiac procedure) (38). Anaerobic bacteria should be considered in patients with concurrent lung abscess, abdominal infection, or history of blunt chest trauma.
TABLE 61.2 Pericarditis: Potential Bacterial Causes | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Importantly, antibiotics alone are not sufficient to treat bacterial pericarditis. All patients require percutaneous or surgical drainage of the pericardial cavity. If the purulent pericardial fluid cannot be percutaneously aspirated, a surgically created window or pericardiectomy will be required (29). Streptokinase administered in the pericardium may improve drainage (39,40). Broad-spectrum antibiotics are mandatory, and initially should be directed toward the most common organisms (Staphylococcus aureus and Haemophilus influenzae). Initial treatment should include intravenous penicillinase-resistant penicillin (nafcillin or oxacillin) or vancomycin in patients at risk for methicillin-resistant Staphylococcus aureus, as well as a third-generation cephalosporin (ceftriaxone, cefotaxime) (34,38,41). An aminoglycoside may be added for immunocompromised patients. Specific therapy can be tailored once specific culture/sensitivity results are known. Patients with bacterial pericarditis should be treated for a minimum of 3 to 4 weeks with intravenous antibiotics.
Survival for patients with bacterial pericarditis is >90% (39,41). Risk factors for poor outcome include young age at diagnosis, septicemia, tamponade, delayed diagnosis, inadequate drainage, concurrent myocarditis, and a staphylococcal etiology (37,39,42). Constrictive pericarditis can be a late complication (36,39), and is most commonly associated with Staphylococcus aureus, Haemophilus influenzae, or Streptococcus pneumoniae infections.
Tuberculous Pericarditis
Once common throughout the world, Mycobacterium tuberculosis pericarditis now occurs most frequently in developing nations. The typical onset is insidious with symptoms of low-grade fever, night sweats, weight loss, malaise, dyspnea, and chest pain. The presentation may be complicated by subacute pericardial tamponade. Tuberculous pericarditis often occurs due to direct extension of miliary tuberculosis or lymphatic spread into the pericardium. Hematogenous spread may occur without evidence of pulmonary infiltrates. Most patients have a positive Mantoux skin test.
The pericardial fluid, when aspirated, is serosanguineous or hemorrhagic with lymphocyte predominance. Acid-fast bacilli may be seen on auramine–rhodamine fluorescent-stained smears (43). Pericardial biopsy is useful to provide histologic confirmation. Pericardial fluid adenosine deaminase levels are diagnostic for tuberculous pericarditis if >50 U/L (26,43,44). Mycobacterium cultures may take up to 6 weeks to grow, and thus treatment should be initiated well before confirmation of diagnosis.
Multidrug therapy is essential due to the risk of drug-resistant tuberculosis. A treatment regime of rifampicin, isoniazid, pyrazinamide, and ethambutol for at least 2 months, followed by isoniazid and rifampicin for another 4 months is highly effective (43). One or two months of steroid therapy may be useful to reduce inflammation and increase pericardial fluid resorption (45). Early in the course of the disease, pericardiectomy is difficult due to the presence of diffuse inflammatory and caseous material. Some investigators recommend delaying pericardiectomy for at least 6 weeks, although this remains controversial (43,46). Tuberculosis is a common cause of chronic pericardial effusion, and constrictive pericarditis may develop after recovery. Worldwide, tuberculous pericarditis is one of the leading causes of constrictive pericarditis (43).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree