Martin M. LeWinter, William E. Hopkins
Pericardial Diseases
Pericardial disease has fascinated physiologists and physicians for hundreds, indeed thousands of years. This is perhaps due to the fact that an organ with no critical function of its own is the site of a wide variety of diseases that result in some of the classic physical findings in cardiology. For more detailed treatment of many individual aspects of pericardial disease than is possible in this chapter the reader is referred to the classic monograph by Ralph Shabetai.1
Anatomy and Physiology of the Pericardium
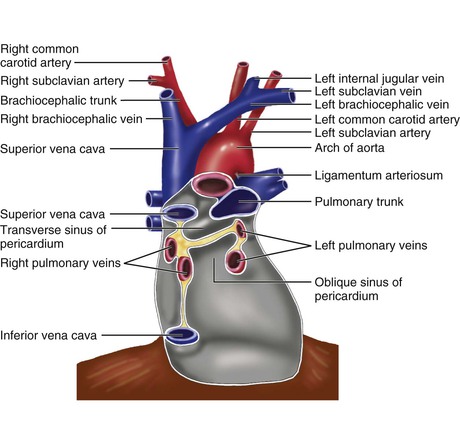
The pericardium is composed of two layers,1 the visceral pericardium, a monolayer of mesothelial cells and collagen and elastin fibers that is adherent to the epicardial surface of the heart, and the fibrous parietal layer, which is approximately 2 mm thick in normal humans and surrounds most of the heart. The parietal pericardium is largely acellular and contains collagen and elastin fibers, with collagen being the major structural component. The visceral pericardium reflects back near the origins of the great vessels and is continuous with and forms the inner layer of the parietal pericardium. The pericardial space or sac is contained within these two layers and normally has up to 50 mL of serous fluid. The visceral pericardium reflects a few centimeters proximal to the junctions of the caval vessels with the right atrium, portions of which lie within the pericardial sac (Fig. 71-1). Posterior to the left atrium the reflection occurs at the oblique sinus of the pericardium. The left atrium is largely extrapericardial. The parietal pericardium has ligamentous attachments to the diaphragm, sternum, and other structures. These attachments ensure that the heart occupies a fixed position within the thoracic cavity. The only noncardiovascular structures associated with the pericardium are the phrenic nerves enveloped by the parietal pericardium.
The Passive Role of the Normal Pericardium in Heart Disease
When the cardiac chambers dilate rapidly, the restraining effect of the pericardium and its contribution to diastolic interaction become augmented, thereby resulting in a hemodynamic picture with features suggestive of both cardiac tamponade and constrictive pericarditis. An example is RV myocardial infarction (MI),1 usually in conjunction with inferior LV MI. Here, the right side of the heart dilates rapidly such that total heart volume exceeds pericardial reserve volume. As a result of the increased pericardial constraint and diastolic interaction, left- and right-sided filling pressures equilibrate at elevated levels and a paradoxical pulse and inspiratory increase in systemic venous pressure (Kussmaul sign) may be observed. Other conditions with similar effects include acute pulmonary embolism and subacute mitral regurgitation.1,2
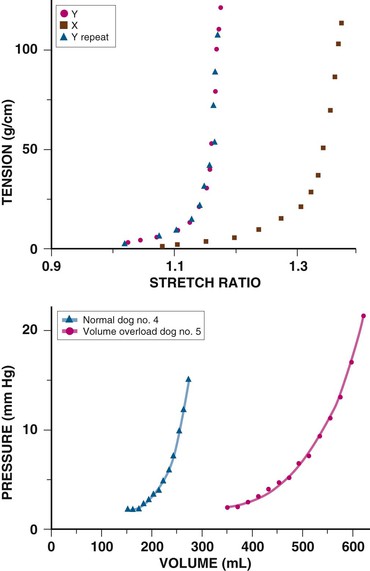
Chronic cardiac dilation, such as in dilated cardiomyopathy or regurgitant valvular disease, can result in cardiac volumes well in excess of the pericardial reserve volume, yet exaggerated restraining effects are not encountered. This implies that the pericardium adapts to accommodate chronic increases in cardiac volume. In experimental chronic volume overload, the pericardial PVR shifts to the right and its slope decreases (Fig. e71-1, bottom, right curve); that is, it becomes more compliant, along with an increase in area and mass and a decreased effect on the LV diastolic PVR.1,2 Presumably, a similar effect occurs with large, slowly accumulating effusions.
Acute Pericarditis
Cause, Epidemiology, and Pathophysiology
Table 71-1 is a partial list of diseases that can involve the pericardium. Acute pericarditis, defined as symptoms and/or signs resulting from pericardial inflammation of no more than 1 to 2 weeks’ duration, can occur in a variety of these diseases (denoted by asterisks), but most cases are considered idiopathic.3,4 We use the term idiopathic to denote acute pericarditis for which no specific cause can be found with routine diagnostic testing as outlined later. Most idiopathic cases are presumed to be viral in cause, but testing for specific viruses is not routine because of cost, low yield, and negligible impact on management.4,5
TABLE 71-1
Categories of Pericardial Disease and Selected Specific Causes
Viral* (echovirus, coxsackievirus, adenovirus, cytomegalovirus, hepatitis B, infectious mononucleosis, HIV/AIDS) Bacterial* (Pneumococcus, Staphylococcus, Streptococcus, Mycoplasma, Lyme disease, Haemophilus influenzae, Neisseria meningitidis, others) Mycobacteria* (M. tuberculosis, M. avium-intracellulare) Fungal (histoplasmosis, coccidiomyocosis) Connective tissue disease* (SLE, RA, scleroderma, dermatomyositis, Sjögren syndrome, mixed) Drug induced* (procainamide, hydralazine, isoniazid, cyclosporine, etc.) Arteritis (polyarteritis nodosa, temporal arteritis) After cardiotomy/thoracotomy,* after cardiac injury* Genetic immune system diseases* (TRAPS, FMF) Primary: mesothelioma, fibrosarcoma, lipoma, etc. Secondary*: breast and lung carcinoma, lymphomas, Kaposi sarcoma Early after cardiac surgery and orthotopic heart transplantation Cholesterol (“gold paint” pericarditis) Chronic renal failure, dialysis associated* Hypothyroidism and hyperthyroidism |

* Causes that can be manifested as the syndrome of acute pericarditis.
AIDS = acquired immunodeficiency syndrome.
The incidence of acute pericarditis is difficult to quantify because, undoubtedly, many cases are undiagnosed. At autopsy, the frequency is approximately 1%.4,6 Pericarditis is common in the emergency department, where it accounts for up to 5% of patients with nonischemic chest pain.4,6 The fraction of all acute cases accounted for by idiopathic pericarditis, as opposed to those with a specific cause identified, is also uncertain and influenced by demographics and variation in viral infections. However, 80% to 90% seems to be a reasonable estimate.4–6 Tuberculous pericarditis is included in Table 71-1 as a cause of acute pericarditis but usually has more chronic symptoms. Bacterial pericarditis is also included because it can be accompanied by signs and symptoms of acute pericardial inflammation, but these patients are generally critically ill and other components of their illness typically dominate. Pericarditis occurring 24 to 72 hours after transmural MI and the delayed pericarditis of Dressler syndrome used to be common (see Chapter 50). However, their incidence has declined during the reperfusion era, and Dressler syndrome is now distinctly rare. Other than this, the causative distribution of acute pericarditis has changed little over time. In contrast, the epidemiology of pericardial effusion and constriction has changed considerably.
The pathophysiology of uncomplicated acute pericarditis is straightforward; that is, the symptoms and signs result from inflammation of pericardial tissue. A minority of cases are complicated, and as many as 15% are associated with myocarditis.4,6–8 Coexistent myocarditis is usually manifested by modest release of biomarkers such as troponin I7,8 (see Chapter 67). LV dysfunction is rare, and the long-term prognosis of pericarditis complicated by myocarditis appears to be excellent.7,8
History and Differential Diagnosis
Acute pericarditis is almost always manifested as chest pain.1,3,4,6 A few cases are diagnosed during evaluation of dyspnea or fever or incidentally in conjunction with noncardiac manifestations of systemic diseases (e.g., systemic lupus erythematosus [SLE]). The pain associated with pericarditis can be quite severe. It is variable in quality but almost always pleuritic. It does not usually have the viselike, constricting, or oppressive features of myocardial ischemia. Pericardial pain typically has a relatively rapid onset. It is commonly substernal but can be centered in the left anterior aspect of the chest or epigastrium. Left arm radiation is not unusual, but the most characteristic is the trapezius ridge, which is highly specific for pericarditis.1 Pericardial pain is relieved by sitting forward and worsened by lying down. Associated symptoms can include dyspnea, cough, and occasionally hiccoughs. An antecedent history suggesting a viral illness is common. It is important to review the past medical history for clues to specific causative diagnoses. A history of cancer or an autoimmune disorder, high fevers with shaking chills, rash, and weight loss are often clues to specific diseases that can cause pericarditis.
The differential diagnosis of chest pain is lengthy (see Chapters 11 and 50). Diagnoses most easily confused with pericarditis include pneumonia with pleurisy, pulmonary embolism/infarction, costochondritis, and gastroesophageal reflux disease. Acute pericarditis is usually relatively easily distinguished from myocardial ischemia, but coronary angiography may be required to resolve the issue. Other considerations include aortic dissection, intra-abdominal processes, pneumothorax, and herpes zoster pain before skin lesions appear. Rarely, pericarditis can signal a preceding, silent MI.
Physical Examination
Patients with uncomplicated acute pericarditis often appear uncomfortable and anxious, with low-grade fever and sinus tachycardia. Otherwise, the only abnormal physical finding is the friction rub caused by contact between the visceral and parietal pericardium. The classic rub is easily recognized and pathognomonic. It consists of three components corresponding to ventricular systole, early diastolic filling, and atrial contraction and has been likened to the sound made when walking on crunchy snow. The rub is usually loudest at the lower left sternal border and best heard with the patient leaning forward. It is often dynamic—disappearing and returning over short periods. Thus frequent auscultation of a patient with suspected pericarditis who does not initially have a rub is often rewarding. Sometimes what is considered a pericardial rub has less than three components. Such sounds should be labeled rubs with caution because they may actually represent a murmur or murmurs. It is important to perform a complete physical examination in a patient with acute pericarditis to look for clues to specific causative diagnoses. The examiner must also be alert to findings indicating significant pericardial effusion, as discussed subsequently.
Laboratory Testing
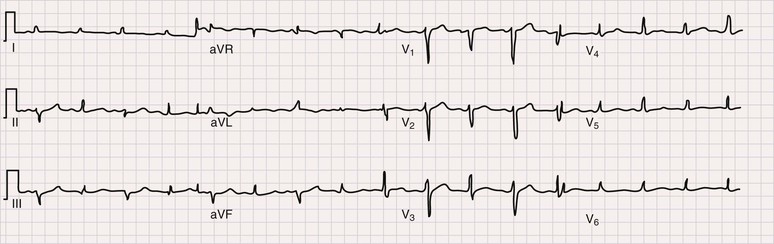
The electrocardiogram (ECG) is the most important test for diagnosing acute pericarditis (see Chapter 12). The classic finding is diffuse ST-segment elevation (Fig. 71-2).1,3,4,6 The ST-segment vector typically points leftward, anterior, and inferior, with ST-segment elevation in all leads except aVR and often V1. Usually, the ST segment is coved upward and resembles the current of injury of transmural ischemia. However, distinction between acute pericarditis and transmural ischemia is not generally difficult because of more extensive lead involvement in pericarditis and much more prominent reciprocal ST depression in ischemia. However, ST elevation in pericarditis can involve a smaller number of leads, thus making distinction more difficult. In some cases the ST segment more closely resembles early repolarization. Here again, pericarditis usually involves more leads than early repolarization does. As with the rub, changes on the ECG can be dynamic. Frequent recordings can yield a diagnosis in patients who initially have neither rub nor ST elevation. PR-segment depression is also common (Fig. 71-2). It can occur without ST elevation and be the initial or sole manifestation of acute pericarditis on the ECG. Abnormalities other than ST elevation and PR depression on the ECG are unusual in patients evaluated soon after the onset of symptoms.
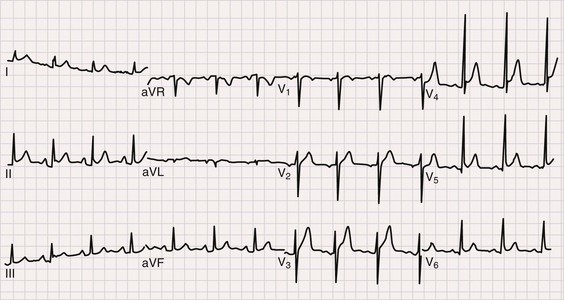
Subsequent ECG changes are variable.1,3,4,6 In some cases the ECG reverts to normal over a period of days or weeks. In others the elevated ST segment passes through the isoelectric point and progresses to ST-segment depression and T wave inversions. These changes can persist for weeks or even months but have no known significance. In patients seen late after the onset of symptoms, the changes can be indistinguishable from myocardial ischemia. Abnormalities on the ECG other than the aforementioned should be considered carefully because they suggest diagnoses other than idiopathic pericarditis and/or the presence of complications. Thus atrioventricular (AV) block may indicate Lyme disease, pathologic Q waves can signify a previous silent MI with pericardial pain as its first manifestation, and low voltage or electrical alternans points toward significant effusion.
The hemogram often reveals a modestly elevated white blood cell count with mild lymphocytosis. A higher count is an alert for other causes, as is anemia.
As noted earlier, as many as 15% of patients with a diagnosis of acute pericarditis have coexistent myocarditis based on elevations in injury biomarkers such as serum troponin I (see Chapter 67). In most cases no LV dysfunction is present. Patients with elevated injury biomarkers almost always have ST-segment elevation.4,6,8 Another concern in patients with increased troponin I is silent MI followed by subsequent pericarditis. Post-MI pericarditis usually occurs after large MIs with transmural changes on the ECG.9
Serum high-sensitivity C-reactive protein (hsCRP) is elevated in approximately three fourths of patients with acute pericarditis.10 Normal values generally occur in patients seen very early or in those who have received anti-inflammatory therapy. In most cases, hsCRP normalizes within 1 week and in almost all cases by 4 weeks after initial evaluation. Increased hsCRP is independently associated with recurrent symptoms. Based on these observations it was suggested10 that serial hsCRP determinations be used to monitor disease activity and aid in determining the duration of therapy. Although the usefulness of hsCRP for this purpose has not been shown prospectively, the association of elevated values with recurrence provides a rationale for measurement of hsCRP at initial encounter and in cases in which it is uncertain how long treatment should be maintained.
Findings on chest radiography are usually normal in uncomplicated acute idiopathic pericarditis. Occasionally, small pulmonary infiltrates or pleural effusions are present, presumably caused by viral or possibly mycoplasmal infections. Other abnormalities suggest alternative diagnoses. Thus bacterial pericarditis often occurs in conjunction with severe pneumonia. Tuberculous pericarditis can develop with or without associated pulmonary disease. Mass lesions and enlarged lymph nodes suggestive of neoplastic disease have great significance. Because small to even moderate pericardial effusions may not cause an abnormal cardiac silhouette, even modest enlargement is of concern.
Findings on the echocardiogram (see Chapter 14) are normal in most patients with acute idiopathic pericarditis. It is obtained mainly to detect an effusion. There are no modern data delineating the incidence of effusions. Small ones are common and not of concern. Moderate or larger effusions are unusual and may signal a diagnosis other than idiopathic pericarditis. Echocardiography is also useful in delineating whether the associated myocarditis is severe enough to alter ventricular function and in detecting MI. It is rarely necessary to use imaging modalities other than echocardiography in the diagnosis and management of uncomplicated acute pericarditis. In difficult cases, computed tomography (CT) and/or cardiac magnetic resonance imaging (MRI) can be helpful in detecting pericardial thickening and, in the case of MRI, in providing evidence of active inflammation based on increased gadolinium uptake.11
Natural History and Management
The European Society of Cardiology published guidelines for the diagnosis and management of pericardial disease in 2004.3 They have been revisited in recent reviews,4,5,12,13 and treatment was addressed in a meta-analysis.14 Although these guidelines and reviews are useful, few randomized trials have been devoted to the diagnosis or management of pericardial disease. It is important to keep in mind that objective data to support recommendations for the management of acute pericarditis, as well as other pericardial diseases, are limited.
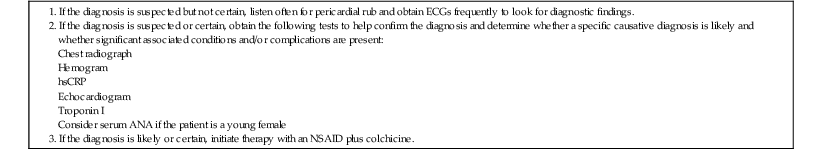
Initial management should be focused on screening for specific causes that would alter management, detection of effusion and other echocardiographic abnormalities, alleviation of symptoms, and appropriate treatment if a specific cause is discovered. Initially, we recommend obtaining the following laboratory data: ECG, hemogram, chest radiograph, troponin I, hsCRP, and an echocardiogram. In young women, testing for SLE is reasonable, but low titers of antinuclear antibody (ANA) are common in patients with recurrent idiopathic pericarditis who do not meet other criteria for SLE.15 Thus the significance of low ANA titers during an initial evaluation is uncertain. Table 71-2 summarizes our recommendations for initial assessment and treatment of patients with definite or suspected acute pericarditis.
TABLE 71-2
Initial Approach to Patients with Definite or Suspected Acute Pericarditis
Acute idiopathic pericarditis is a self-limited disease without significant complications or recurrence in 70% to 90% of patients.3,4,6,16 If laboratory data do not contradict the clinical diagnosis of idiopathic pericarditis, symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) should be initiated.3,4,6,12–14 Because of its excellent safety profile, we prefer ibuprofen (600 to 800 mg orally three times daily). Acetylsalicylic acid (ASA), 2 to 4 g daily in divided doses, is an alternative and often preferable in patients who require ASA for other indications. In either case, gastric protection in the form of a proton pump inhibitor should be provided.
Many patients have gratifying responses to the first dose or two of an NSAID. Most respond fully after 10 to 14 days and need no additional treatment. As noted, using normalization of hsCRP to guide the duration of therapy10,13 is a reasonable alternative to a predetermined time course. Reliable patients with no more than small effusions who respond well to initial therapy need not be admitted to the hospital. Those who do not respond well initially, who have larger effusions, or in whom a cause other than idiopathic pericarditis is suspected should be hospitalized for additional observation, diagnostic testing, and treatment as necessary.
Over the last decade the use of colchicine for acute idiopathic pericarditis has become established. Originally, colchicine was found to be effective in preventing recurrences or in treating pericarditis that did not respond well initially to NSAIDs.3,16 There is now reasonable evidence, including randomized trials, to support its use as part of the initial therapy for acute idiopathic pericarditis.4,6,12–14,17,18 Colchicine improves the initial response when used in conjunction with an NSAID and reduces the chance of recurrence. The drug is thought to exert an anti-inflammatory effect by blocking microtubule assembly in white blood cells.13 Colchicine is administered at a dose of 0.5 mg twice daily in patients heavier than 70 kg and 0.5 mg daily in patients less than 70 kg, with dose reduction in those with impaired renal function. These doses are generally very well tolerated, although gastrointestinal side effects result in withdrawal in approximately 10% of patients. In those who respond slowly to an NSAID and colchicine, narcotic analgesics may be used to allow time for a more complete symptomatic response. Because prevention of recurrences is a major rationale, we recommend 3 months of colchicine therapy following an initial episode.13,19
Patients who respond poorly to NSAIDs have typically been treated with corticosteroids. However, their use appears to promote recurrences.6,12–14,18,20 Moreover, the previous recommendation of short courses of high-dose corticosteroids with a rapid taper seems to especially increase recurrences.20,21 Lower initial doses with more gradual tapering appear to be preferable with respect to both recurrences and adverse effects. If corticosteroids cannot be avoided in managing an initial episode, we prefer 0.2 to 0.5 mg/kg/day of prednisone initially followed by tapering every 2 to 4 weeks. Tapering should be guided by symptomatic response and serial measurement of hsCRP. We recommend concurrent colchicine during prednisone therapy. In view of the potential for increased side effects, especially gastrointestinal, we discontinue NSAIDs when prednisone is started, with gradual reinstitution during tapering.
Complications of acute pericarditis include effusion, tamponade, and constriction (myocarditis is an associated condition rather than a complication). As noted earlier, small effusions are common. Relatively little is known about the incidence of more significant complications. In the largest modern report,22 a specific cause was identified in 17% of patients with acute pericarditis. Over an average 31-month follow-up, tamponade developed in 3.1% and constriction in 1.5%. Most complications occurred in patients with identified causes. Constrictive pericarditis was addressed in more detail in a recent analysis of 500 patients.23 Overall, constriction developed in 1.8% over a median 72-month follow-up. In the 83% of patients with idiopathic/viral pericarditis, constriction developed in only 0.48%. Thus in patients with idiopathic/viral pericarditis, the incidence of complications is very low.
Relapsing and Recurrent Pericarditis
Perhaps 15% to 30% of patients with apparent idiopathic acute pericarditis who respond satisfactorily to treatment suffer a relapse.4,6,12,13,16 Women and those who initially fail treatment with NSAIDs are at increased risk.16 Recurrent bouts of pain develop in a minority and can be very debilitating. Recurrent pain is not necessarily associated with objective signs of inflammation. Some patients with what is initially thought to be idiopathic pericarditis have evidence of a specific cause as recurrences develop. Accordingly, repeated evaluation for specific causes is appropriate. Pericardial biopsy to look for a specific cause in patients with recurrent pain without effusion is rarely indicated because it is unlikely that a diagnosis will actually be determined or the information obtained will alter management. The rate of complications, including constriction, is very low in patients with recurrent idiopathic pericarditis, and the long-term prognosis is good, with most eventually having full remission.21,24
Genetic disorders of the immune system underlie some cases of recurrent pericarditis. A recent study found that 8 of 131 (6.1%) patients thought to have recurrent idiopathic pericarditis had mutations of the TNFRSF1A gene that cause tumor necrosis factor receptor-1–associated periodic syndrome (TRAPS),25 a monogenic disorder resulting in dysfunction of the innate immune system with periodic fever, rash, abdominal pain, periorbital edema, and polyserositis with pericarditis.26 Patients with TRAPS respond to corticosteroids but not to colchicine. In another report,27 4 of 30 patients with recurrent pericarditis refractory to colchicine were found to have TNFRSF1A mutations. A report that human leukocyte antigen allele patterns are associated with recurrent pericarditis28 also supports a role of genetic variation in innate immunity as an underlying determinant. The extent to which TRAPS and other innate immune disorders are responsible for recurrent pericarditis merits additional research. Symptoms consistent with TRAPS should be sought in patients with recurrent pericarditis.
Treatment of recurrent pericardial pain is empiric. For an initial relapse, a repeated course of an NSAID is often effective.13,16 Here again, monitoring hsCRP levels may be useful in guiding therapy. Colchicine is effective for both treatment and prophylaxis of recurrent pain. Thus if colchicine therapy has not previously been initiated, it should certainly be started with a recurrence and be continued for 6 to 12 months in the doses recommended earlier. Many patients with recurrent pericarditis find that episodes can be managed with reinstitution of NSAID therapy at the first sign of symptoms. Patients with recurrent pericardial pain despite NSAIDs and colchicine are a challenging problem. In these cases, prednisone therapy as outlined earlier can be used. Individualization of therapy is critical. Thus despite our general aversion to brief courses of high-dose corticosteroids, in occasional patients with recurrent pericarditis such an approach may be the only practical way to provide relief of symptoms without long-term side effects. Nonsteroidal immunosuppressive drugs such as azathioprine and cyclophosphamide are another alternative, but no systematic experience is available for guidance. Pericardiectomy has occasionally been performed for recurrent pericarditis. A recent report in children29 was somewhat encouraging, and we have encountered occasional patients with a satisfactory response, but pericardiectomy should be reserved for those whose quality of life is severely affected, who are clearly medical treatment failures, and who desire this option despite the fact that remission is likely to occur in the long term.
Pericardial Effusion and Tamponade
Cause
Idiopathic pericarditis and any infection, neoplasm, or autoimmune or inflammatory process that can cause pericarditis can cause an effusion (see Table 71-1).1,3–4 Effusions are common early after routine cardiac surgery and orthotopic heart transplantation,30 but tamponade is unusual, and they generally resolve within several weeks to a few months. A lengthy list of miscellaneous, noninflammatory diseases can cause effusion (see Table 71-1). Patients with severe circulatory congestion may have small to moderate transudative effusions. Bleeding into the pericardial sac occurs after blunt and penetrating trauma (see Chapter 72), following post-MI rupture of the free wall of the left ventricle, and as a complication of percutaneous cardiac procedures. Retrograde bleeding is an important cause of death from aortic dissection (see Chapter 57). Last, occasional patients are encountered with large, silent pericardial effusions.31 These effusions are generally stable, but instances of tamponade do occur over time.
Causes of effusion with a high incidence of progression to tamponade include bacterial, fungal, and human immunodeficiency virus (HIV)-associated infections (see Chapter 70); bleeding; and neoplastic involvement. Although large effusions attributable to acute idiopathic pericarditis are unusual, because it is so common, this form of pericarditis accounts for a significant percentage of tamponade cases. Approximately 20% of large, symptomatic effusions without an obvious cause following routine evaluation constitute the initial manifestation of a cancer.32 Details of pericardial effusion pertinent to specific disease entities are discussed in the sections devoted to these diseases at the end of this chapter.
Pathophysiology and Hemodynamics
Formation of an effusion is a component of the inflammatory response when an inflammatory, infectious, or neoplastic process is affecting the pericardium. Lymphomas occasionally cause effusion in association with enlarged mediastinal lymph nodes1 by obstructing lymph drainage. The pathophysiology of effusions in situations with no obvious inflammation, such as uremia or idiopathic, is very poorly understood.
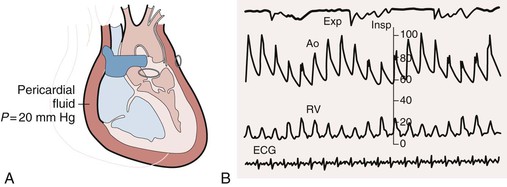
Cardiac tamponade comprises a continuum from an effusion causing minimal effects to one causing full-blown circulatory collapse. Clinically, the most critical point occurs when an effusion reduces the volume of the cardiac chambers such that cardiac output declines. Determinants of the hemodynamic consequences of an effusion are the pressure in the pericardial sac and the ability of the heart to compensate. The pressure in turn depends on the amount of fluid and the pericardial PVR. As discussed earlier, the pericardium normally has little reserve volume. As a result, relatively modest amounts of rapidly accumulating fluid (as little as 150 to 200 mL) can have major effects on cardiac function. Large, slowly accumulating effusions are often well tolerated, presumably because of chronic changes in the pericardial PVR described earlier. The compensatory response to an effusion includes increased adrenergic stimulation and parasympathetic withdrawal, which causes tachycardia and increased contractility1 and can maintain cardiac output and blood pressure for a period. Eventually, however, cardiac output and blood pressure decline. Patients who cannot mount a normal adrenergic response, such as those receiving beta-blocking drugs, are more susceptible to the effects of an effusion. In terminal tamponade, a depressor reflex with paradoxical bradycardia may supervene.
Clinical Features
Obviously, in patients with pericardial effusions a history pertinent to a specific cause may be elicited. Occasionally, large, asymptomatic chronic effusions are discovered when a chest imaging study is performed for an unrelated reason.31 As discussed, specific causes are not usually found in these cases. Effusions do not in and of themselves cause symptoms unless tamponade is present, although many patients have pain because of associated pericarditis. Patients with tamponade may complain of dyspnea, whose mechanism is uncertain because of the absence of pulmonary congestion. They are almost always more comfortable sitting forward. Other symptoms reflect the severity of reduction in cardiac output and blood pressure. Pericardial pain and/or a nonspecific discomfort may dominate the clinical picture.
A complete physical examination of patients with pericardial effusion may provide clues to a specific cause. In pericardial effusion without tamponade, findings on cardiovascular examination are normal; however, if the effusion is large, the cardiac impulse may be difficult to palpate and the heart sounds are muffled. A friction rub may of course also be present. Tubular breath sounds may be heard in the left axilla or base because of bronchial compression. Beck’s triad—hypotension, muffled heart sounds, and elevated jugular venous pressure—remains a useful clue to the presence of severe tamponade. Patients with tamponade appear uncomfortable, with signs reflecting varying degrees of reduced cardiac output and shock, including tachypnea, diaphoresis, cool extremities, peripheral cyanosis, depressed sensorium, and rarely, yawning.1 Hypotension with reduced pulse pressure is usually present, although in the early stages compensatory mechanisms maintain blood pressure. Some patients with subacute tamponade are hypertensive on initial evaluation,40 with a decline in blood pressure occurring with pericardial drainage. A paradoxical pulse is the rule, but it is important to be alert to situations in which it may be absent. The paradox is quantified by cuff sphygmomanometry as the difference between the pressure at which Korotkoff sounds first appear and that at which they are present with each contraction. In severe tamponade the inspiratory decrease in arterial pressure is palpable and greatest in arteries distant from the heart. Tachycardia is also the rule unless heart rate–lowering drugs have been administered, conduction system disease coexists, or a preterminal bradycardic reflex has supervened. Jugular venous pressure is markedly elevated except in low-pressure tamponade, and the y descent is usually absent (see Fig. 71-4). The normal decrease in venous pressure on inspiration is retained. Examination of the heart itself is simply consistent with an effusion, as outlined earlier. Tamponade can be confused with anything that causes hypotension, shock, and elevated jugular venous pressure, including myocardial failure, right-sided heart failure because of pulmonary embolism or other causes of pulmonary hypertension, and RV MI.
Laboratory Testing
Abnormalities of effusion and tamponade on the ECG are reduced voltage and electrical alternans1,3,4,6 (Fig. 71-6). Reduced voltage is nonspecific and can be caused by conditions such as emphysema, infiltrative myocardial disease, and pneumothorax. Electrical alternans is specific but relatively insensitive for large effusions. It is caused by anterior-posterior swinging of the heart with each contraction. When pericarditis coexists, the usual findings on the ECG may be present.
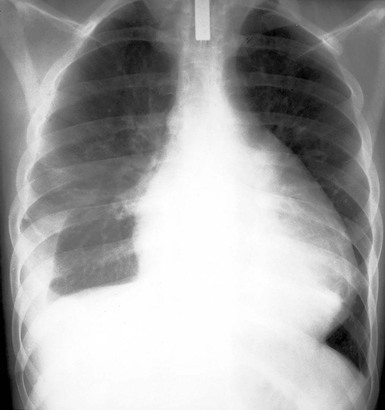
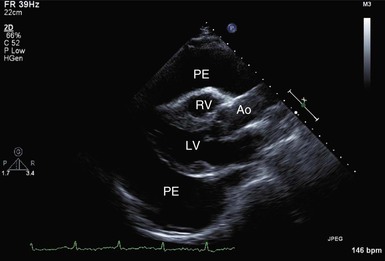
The chest radiograph reveals a normal cardiac silhouette until the effusions are at least moderate in size. With larger effusions the anteroposterior cardiac silhouette assumes a rounded, flasklike appearance (Fig. e71-2![]() ). Lateral views may reveal the fat pad sign, a linear lucency between the chest wall and the anterior surface of the heart caused by separation of parietal pericardial fat from the epicardium. The lungs appear oligemic.
). Lateral views may reveal the fat pad sign, a linear lucency between the chest wall and the anterior surface of the heart caused by separation of parietal pericardial fat from the epicardium. The lungs appear oligemic.
M-mode and two-dimensional Doppler echocardiographic techniques remain the standard noninvasive methods for detection of pericardial effusion and tamponade. An effusion appears as a lucent separation between the parietal and visceral pericardium for the entire cardiac cycle (Fig. 71-7 and Video 71-1![]() ). Small effusions are first evident over the posterobasal part of the left ventricle. As the fluid increases, it spreads anteriorly, laterally, and behind the left atrium, where its limit is demarcated by the visceral pericardial reflection. Ultimately, the separation becomes circumferential. Circumferential effusions are graded as small (echo-free space in diastole <10 mm), moderate (10 to 20 mm), and large (>20 mm). Because the rapidity of accumulation is critical, the hemodynamic significance of an effusion does not always correlate closely with its size. However, tamponade does not usually occur without a circumferential effusion, and the diagnosis should be viewed skeptically if such is not the case. However, as discussed, loculated effusions can cause tamponade. Frondlike or shaggy-appearing structures in the pericardial space detected on echocardiography suggest clots, chronic inflammation, or neoplastic pericardial processes. CT and MRI are more precise than echocardiography in imaging the pericardium itself.
). Small effusions are first evident over the posterobasal part of the left ventricle. As the fluid increases, it spreads anteriorly, laterally, and behind the left atrium, where its limit is demarcated by the visceral pericardial reflection. Ultimately, the separation becomes circumferential. Circumferential effusions are graded as small (echo-free space in diastole <10 mm), moderate (10 to 20 mm), and large (>20 mm). Because the rapidity of accumulation is critical, the hemodynamic significance of an effusion does not always correlate closely with its size. However, tamponade does not usually occur without a circumferential effusion, and the diagnosis should be viewed skeptically if such is not the case. However, as discussed, loculated effusions can cause tamponade. Frondlike or shaggy-appearing structures in the pericardial space detected on echocardiography suggest clots, chronic inflammation, or neoplastic pericardial processes. CT and MRI are more precise than echocardiography in imaging the pericardium itself.
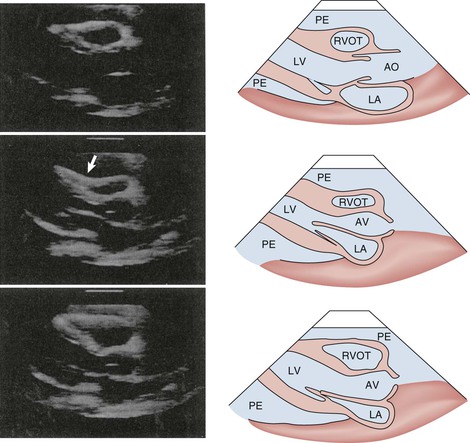
As discussed previously, tamponade is best considered as a spectrum of severity of cardiac compression. Several echocardiographic findings indicate that tamponade is severe enough to cause hemodynamic compromise,34–36 including early diastolic collapse of the right ventricle, late diastolic indentation or collapse of the right atrium, exaggerated variation in RV and LV chamber size, and interventricular septal shifting during inspiration. Early diastolic collapse of the right ventricle (Fig. 71-8; see also Video 71-1) and late diastolic collapse of the right atrium (both of which occur during ventricular diastole) (Fig. 71-9; see also Video 71-1) are reported to be sensitive and specific signs that appear relatively early during tamponade.34–36 Both occur when pericardial pressure transiently exceeds intracavitary pressure. In our experience, however, late diastolic collapse of the right atrium can be confused with right atrial systole. The cardiac chambers are small in tamponade, and as discussed previously, the heart may swing anteroposteriorly (see Video 71-1). Distention of the caval vessels that does not diminish with inspiration is also a useful sign. A large pleural effusion can cause right-sided chamber collapse.38 Isolated chamber collapse/compression, including the left ventricle and atrium, can occur with pericardial hematomas after cardiac surgery.35