Percutaneous Valve Dilatations
Alec S. Vahanian
Overview
More than 20 years of experience with percutaneous valve dilatation for acquired valve stenoses have shown the following:
Percutaneous mitral valvuloplasty is an effective treatment in a wide range of patients with mitral stenosis. It is a low-risk procedure when it is performed by experienced teams, and follow-up at up to 15 years demonstrates excellent durability of the procedure.
Percutaneous aortic valvuloplasty (PAV) for degenerative calcified aortic stenosis provides short-term palliation of symptoms at the cost of high periprocedural risk. Its role is in question.
Percutaneous tricuspid or multivalve dilatation is used in exceptional cases.
Percutaneous dilatation of a bioprosthesis has no future.
Historical Perspective
Until the early 1980s, surgery was the only possible treatment for severe valvular stenoses. Then a new alternative appeared: percutaneous balloon valvuloplasty. The first to perform balloon valvuloplasty in the treatment of mitral stenosis was K. Inoue in 1982 (1). In the field of aortic stenosis, percutaneous valvuloplasty was used for the first time by Cribier et al. in 1985 (2).
This chapter deals with percutaneous balloon valvuloplasty for acquired valvular stenoses in patients with mitral stenosis, aortic stenosis, and the less common tricuspid, bioprosthetic, and multivalvular stenoses.
Percutaneous Mitral Valvuloplasty
Rheumatic fever continues to be endemic in developing countries, where mitral stenosis is the most common valve disease. Although the prevalence of rheumatic heart disease has greatly decreased in industrialized countries, this disease continues to represent an important clinical entity because of outmigration from developing countries and the presence of restenosis after previous surgical commissurotomy (3,4).
Mechanisms
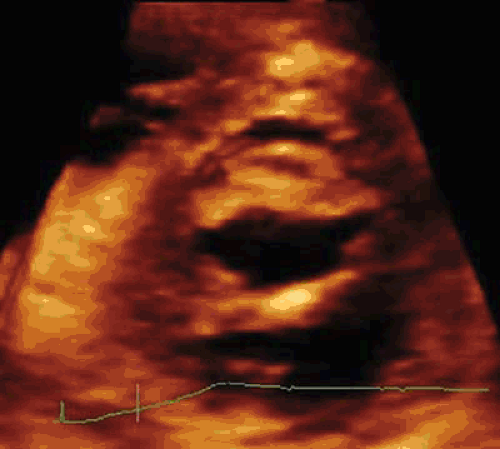
Balloon dilatation acts in the same way as surgical commissurotomy by opening the fused commissures; therefore, a more appropriate term for the procedure is probably percutaneous mitral commissurotomy rather than percutaneous mitral valvuloplasty (5) (Fig. 82.1). Balloons are also able to enlarge the valve area by fracturing nodular deposits in patients with calcified valves (6).
Technique
The transvenous or antegrade approach is the most widely used. Transseptal catheterization, which allows access to the left atrium, is the first step in the procedure and one of the most crucial. The retrograde technique without transseptal catheterization, in which the balloon is introduced through the femoral artery, is very seldom used today (7).
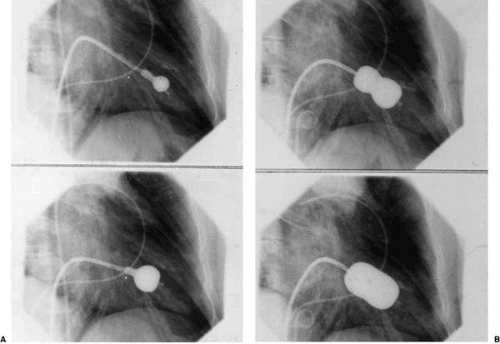
The Inoue technique (Fig. 82.2), the first to be developed (1,8), is used almost exclusively. The Inoue balloon is made
of nylon and rubber micromesh, and it is self-positioning and pressure extensible. The balloon has three distinct parts, each with a specific elasticity, which can be inflated sequentially. The Inoue balloon comes in four sizes, ranging from 24 to 30 mm, and each is pressure dependent, so its diameter can be varied by up to 4 mm as required by circumstances. Inoue recommends the use of a stepwise dilatation technique under echocardiographic guidance: the first inflation is performed to the minimal diameter of the balloon chosen. The balloon is then deflated and is withdrawn into the left atrium. If mitral regurgitation has not increased and valve area is insufficient, the balloon is readvanced across the mitral valve, and inflation is repeated with the balloon diameter increased by 1 to 2 mm.
of nylon and rubber micromesh, and it is self-positioning and pressure extensible. The balloon has three distinct parts, each with a specific elasticity, which can be inflated sequentially. The Inoue balloon comes in four sizes, ranging from 24 to 30 mm, and each is pressure dependent, so its diameter can be varied by up to 4 mm as required by circumstances. Inoue recommends the use of a stepwise dilatation technique under echocardiographic guidance: the first inflation is performed to the minimal diameter of the balloon chosen. The balloon is then deflated and is withdrawn into the left atrium. If mitral regurgitation has not increased and valve area is insufficient, the balloon is readvanced across the mitral valve, and inflation is repeated with the balloon diameter increased by 1 to 2 mm.
In developing countries, economic constraints lead to a limited residual use of multitrack balloons (9), a variant of the double-balloon technique, and the use of a reusable metallic commissurotome (10). Balloon size is usually chosen according to patient characteristics: height and body surface area (8,11,12).
Monitoring of the Procedure and Assessment of Immediate Results
Two methods are used to assess immediate results in the catheterization laboratory: hemodynamic testing and echocardiography. Although echocardiography may be difficult to perform in the catheterization laboratory for logistic reasons, it
provides essential information on the efficacy of the procedure and also enables early detection of complications. The following criteria have been proposed for the desired end point of the procedure: (a) mitral valve area greater than 1 cm2/m2 body surface area, (b) complete opening of at least one commissure, or (c) appearance or increment of regurgitation greater than 1/4 classification (8).
provides essential information on the efficacy of the procedure and also enables early detection of complications. The following criteria have been proposed for the desired end point of the procedure: (a) mitral valve area greater than 1 cm2/m2 body surface area, (b) complete opening of at least one commissure, or (c) appearance or increment of regurgitation greater than 1/4 classification (8).
TABLE 82.1 Valve Area after Percutaneous Mitral Valvuloplasty | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||
After the procedure, the most accurate evaluation of valve area is provided by planimetry using echocardiography (13). Tailoring the strategy to the individual circumstances is vital; clinical factors as well as anatomic factors and the cumulative data of periprocedural monitoring should be taken into account. For example, balloon size, increments of size, and expected final valve area are smaller in elderly patients, in patients with very tight mitral stenosis or extensive valve and subvalvular disease, and in patients with nodular commissural calcification.
In addition, echocardiography using the transesophageal approach, or more recently intracardiac probes, may facilitate the procedure, especially transseptal puncture (14,15). To allow for the slight loss in valve area that occurs during the first 24 hours, echocardiography should be performed 1 or 2 days after mitral valvuloplasty, when calculation of the valve area may be done by planimetry, the half-pressure time method, or the continuity equation method. The final assessment of the degree of regurgitation may be made by angiography or by Doppler color flow imaging. Transesophageal examination is recommended in patients with severe mitral regurgitation to determine the mechanisms involved. The most sensitive method for the assessment of shunting is Doppler color flow imaging, especially when transesophageal examination is used, because this type of Doppler imaging shows the severity of the defect and detects shunting in a more sensitive way than does assessment of hemodynamics.
Immediate Results
The technique of mitral valvuloplasty has been evaluated in several thousand patients with different clinical conditions and valve anatomy (7,9,10,16,17,18,19,20,21,22,23).
Efficacy
The results shown in Table 82.1 demonstrate that mitral valvuloplasty usually provides an increase of more than 100% in valve area. Overall good immediate results, defined by a final valve area greater than 1.5 cm2 without mitral regurgitation greater than 2/4, are observed in more than 80% of cases.
Failures
Risks
Procedural mortality ranges from 0% to 3% (7,9,10,16,17,18,19,20,21,22,23) (Table 82.2). The main causes of death are left ventricular perforation and poor general condition of the patient. The incidence of hemopericardium varies from 0.5% to 12%. Pericardial hemorrhage may be related to transseptal catheterization or to apex perforation by the guidewires or the balloon itself when one uses the double-balloon technique. Embolism is encountered in 0.5% to 5% of cases. The frequency of severe mitral regurgitation ranges from 2% to 19% (24,25,26). Surgical findings (26,27) have shown that this condition is mostly related to noncommissural leaflet tearing, which could be associated with chordal rupture. The development of severe mitral regurgitation depends more on the distribution of the morphologic changes of the valve than on their severity (6,28). Severe mitral regurgitation may be well tolerated, but more often it is not, and surgery on a scheduled basis is necessary. In most cases, valve replacement is required because of the severity of the underlying valve disease. Conservative surgery has been successfully performed in patients with less severe valve deformity (26). The frequency of atrial septal defect reported after valvuloplasty varies from 10% to 90%, depending on the
technique used for its detection (29,30). These shunts are usually small and without clinical consequences. Although urgent surgery (within 24 hours) is seldom needed for complications, it may be required for massive hemopericardium resulting from left ventricular perforation intractable to treatment by pericardiocentesis or, less frequently, for severe mitral regurgitation with poor hemodynamic tolerance (21,22,23,31).
technique used for its detection (29,30). These shunts are usually small and without clinical consequences. Although urgent surgery (within 24 hours) is seldom needed for complications, it may be required for massive hemopericardium resulting from left ventricular perforation intractable to treatment by pericardiocentesis or, less frequently, for severe mitral regurgitation with poor hemodynamic tolerance (21,22,23,31).
TABLE 82.2 Major Complications of Percutaneous Mitral Valvuloplasty | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 82.3 Follow-up after Percutaneous Mitral Valvuloplasty | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
Predictors of Immediate Results
The prediction of results is multifactorial (22,32,33). Several studies have shown that, in addition to morphologic factors (22,32,33,34,35,36), preoperative variables such as age, history of surgical commissurotomy, functional class, small mitral valve area, presence of mitral regurgitation before valvuloplasty, atrial fibrillation, high pulmonary artery pressure, and presence of severe tricuspid regurgitation, as well as procedural factors such as balloon type and size, are all independent predictors of the immediate results. The identification of variables linked to outcome enabled the development of predictive models, from which it appears that the sensitivity of prediction is high. Nevertheless, specificity is low, indicating insufficient prediction of poor immediate results. This latter finding is particularly true with regard to the lack of accurate prediction of severe mitral regurgitation. This low specificity is related to the intrinsic limitations of the prediction of immediate results, that is, to the possibility of good results in patients who are at high risk for poor results. The possibility of good results in theoretically unsuitable cases has been demonstrated in experimental studies and confirmed clinically.
Long-Term Results
Data from follow-up of up to 15 years can now be analyzed. In clinical terms, the overall long-term results of valvuloplasty are good (36,37,38,39,40,41,42,43,44,45,46) (Table 82.3). Late outcome after valvuloplasty differs according to the quality of the immediate results (Fig. 82.3).
When the immediate results are unsatisfactory, patients experience only transient or no functional improvement, and delayed surgery is usually performed when extracardiac conditions allow.
Conversely, if valvuloplasty is initially successful, then survival rates are excellent, functional improvement occurs in the majority of cases, and the need for secondary surgery is infrequent (37,42). When clinical deterioration occurs in these patients, it is late and mainly related to mitral restenosis. Determining the incidence of restenosis by echocardiography is compromised by the absence of a uniform definition. Restenosis has generally been defined as a loss of more than 50% of the initial gain, with a valve area becoming smaller than 1.5 cm2. After a successful procedure, the incidence of echographically identified restenosis is usually low, ranging from 2% to 40% at time intervals of 3 to 5 years (36,40,41,42,43,44). The possibility of repeat valvuloplasty in patients with recurrent mitral stenosis is one of the potential advantages of this nonsurgical procedure. Repeated valvuloplasty can be proposed if recurrent stenosis leads to symptoms, if it occurs several years after an initially successful procedure, and if the predominant mechanism of restenosis is commissural refusion (45,46




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree