Percutaneous Structural Heart Disease Procedures
Shikhar Agarwal
Amar Krishnaswamy
The last decade witnessed a remarkable rise in the number of novel percutaneous, catheter-based procedures using new concepts and technologies for the treatment of structural heart disease. Most percutaneous therapies have largely evolved from concepts developed by surgeons and often incorporate the lessons learned from established surgical procedures. Currently, percutaneous catheter-based therapy is available for a number of valvular disorders, including aortic stenosis (AS), mitral stenosis (MS), mitral regurgitation (MR), and prosthetic paravalvular leaks (PVLs), as well as other structural cardiac disorders such as hypertrophic obstructive cardiomyopathy (HOCM), patent foramen ovale (PFO), and atrial septal defects (ASDs). While a comprehensive review of structural cardiac interventions is beyond the scope of this chapter, we provide an overview of the major devices and clinical studies that have been performed.
I. PERCUTANEOUS TRANSCATHETER MITRAL VALVE REPAIR (MVRE)
A. Background.
MR is one of the most common valvular disorders, encountered in about 7% of the population aged > 75 years. MVRe, when feasible, produces superior outcomes, including lower operative mortality, improved long-term survival, and reduced incidence of endocarditis and thromboembolic complications, compared with mitral valve replacement (MVR). A number of percutaneous MVRe techniques have been developed that are analogous to surgical procedures. Percutaneous mitral annuloplasty leverages the close relationship of the coronary sinus (CS) to the posterior mitral annulus. The Carillon (Cardiac Dimensions, Kirkland, WA) device, which has been granted Conformité Européenne (CE Mark) approval, is deployed in the CS and serves to “cinch” the annulus, resulting in improved mitral valve (MV) leaflet coaptation. There are also numerous other devices in various stages of preclinical and clinical development.
The MitraClip (Abbott Vascular, Santa Clara, CA) remains the most widely tested system in humans and is currently the only device available (under trial basis) in the United States. A total of more than 4,000 procedures have been performed in the United States (under trial) and Europe (where the device has CE Mark approval). The technique is based loosely on the surgical Alfieri stitch, which brings the anterior and posterior leaflets in close apposition using a suture and creates an anatomic “double-orifice” MV.
B. Procedure.
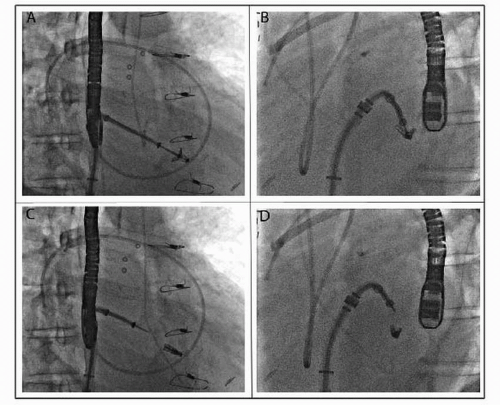
The MitraClip system uses a steerable 22F guide catheter that is introduced through the femoral vein and subsequently advanced to the left atrium via transseptal puncture (Fig. 66.1). Through this guiding catheter, a delivery system containing the V-shaped clip is introduced in the left atrium and positioned with the arms of the clip perpendicular to the MV line of coaptation using transesophageal echocardiography (TEE) guidance. The clip is advanced to the left ventricle in an open position and retracted to grasp the anterior and posterior MV leaflets at the desired location. After confirmation by TEE of clip position, the clip can be locked.
If satisfactory (by TEE), the clip can be released; otherwise, it can be reopened and the process repeated. If necessary, multiple clips can be deployed to achieve a satisfactory result. Care must be taken in this situation to not impede forward flow across the valve and trade MR for MS.
If satisfactory (by TEE), the clip can be released; otherwise, it can be reopened and the process repeated. If necessary, multiple clips can be deployed to achieve a satisfactory result. Care must be taken in this situation to not impede forward flow across the valve and trade MR for MS.
C. Complications.
Routine complications associated with vascular access like major or minor bleeding are the most commonly encountered complications. Recurrent MR and the requirement for MV surgery are the prime limitations of the current technique, especially in ischemic MR or in the presence of a significant annular calcification. Partial clip detachment is the most important mechanical problem encountered with the procedure. These are generally not symptomatic and are treatable with MV surgery or placement of an additional clip. Iatrogenic MS is a significant complication that may arise as a result of this procedure. Careful echocardiographic evaluation of the MV apparatus is important prior to leaflet plication. A coaptation length of at least 2 mm, a baseline MV area of at least 4 cm2, and central origin of the regurgitant jet should be ensured prior to the percutaneous repair, to maximize success and avoid complications.
D. Outcomes.
The edge-to-edge repair technique was tested in the EVEREST I (Endovascular Valve Edge-to-Edge REpair Study) safety and feasibility trial, which demonstrated a 74% procedural success rate (reduction in MR to ≤ 2+) with a < 1% inpatient mortality rate. Freedom from death, MV surgery, or MR > 2+ was 66% at the end of 1 year. Data from the pivotal EVEREST II trial, which randomized 279 patients to MitraClip versus surgical repair in a 2:1 fashion, demonstrated freedom from the combined end point (death, MV surgery within 90 days, or MR > 2+ at
1 year) of 72.4% and 87.8% in the two groups, respectively, confirming noninferiority of the MitraClip to conventional surgical treatment.
1 year) of 72.4% and 87.8% in the two groups, respectively, confirming noninferiority of the MitraClip to conventional surgical treatment.
The initial studies of the MitraClip concentrated on patients with degenerative disease. However, the EVEREST II trial and the clinical experience in Europe (where the device enjoys the CE Mark) have shown efficacy in a large number of patients with functional MR as well. Freedom from significant MR was enjoyed by more than 80% of high surgical risk patients, with resultant decreases in left ventricular (LV) volume, NYHA class, congestive heart failure (CHF) hospitalizations, and improved quality of life. Percutaneous MVRe procedures still remain investigational in the United States and in the current iterations available are applicable to patients in whom surgery is of relatively high risk, given the excellent outcomes with surgical MVRe in terms of both freedom from reoperation and significant MR and operative morbidity and mortality.
II. PERCUTANEOUS MITRAL BALLOON VALVULOPLASTY (PMBV)
A. Background.
Although there has been a significant reduction in the prevalence of rheumatic heart disease in western countries, it still represents a major public health concern in the developing world. MS is one of the most common presentations of rheumatic heart disease. It continues to represent a major clinical problem in the United States primarily due to outmigration from developing countries or the occurrence of restenosis after previous surgical commissurotomy. Stenosis of the valve may occur due to commissural fusion, leaflet thickening, and/or chordal shortening and fusion.
Before the advent of PMBV, symptomatic MS was treated using surgical commissurotomy. Since the introduction of the percutaneous procedure in the early 1980s by Inoue and colleagues, PBMV has evolved to become the first line of therapy for appropriately selected patients with MS. The technique works similarly to commissurotomy, resulting in opening of the fused commissures.
B. Procedure. The Wilkins splitability score is the most widely used echocardiographic parameter to determine the safety and feasibility of PBMV, and this takes into consideration leaflet mobility, leaflet thickening, subvalvular thickening, and valve calcification (each scored 1 to 4 points). Multiple investigators have shown that patients with a score of 8 or less have the greatest freedom from death and valve surgery and the largest improvement in mitral valve area with PMBV.
Selection of the appropriate balloon size is one of the most important steps for accomplishment of a successful PBMV. A good rule of thumb is to use the following formula to determine the maximum balloon dilation size: Balloon size (mm) = patient height (cm)/10 + 10 mm. Sizing of the balloon with contrast inflation is confirmed ex vivo using a measurement device (provided). We routinely perform the procedure as follows: a 5F arterial access is obtained in order to obtain left ventriculography to understand the position of the MV and commissural anatomy in the fluoroscopic projection. Transseptal puncture is performed (most commonly with TEE guidance) using the standard Brockenbrough needle via a 9F Mullins sheath placed in the femoral vein. Once the sheath has entered the left atrium, it is exchanged for the Inoue balloon catheter.
The balloon catheter is slowly advanced into position in the left ventricle. The Inoue balloon consists of three portions with slightly different compliances. As pressure is added to the balloon, the distal portion inflates first followed by the proximal portion. As soon as the distal portion is inflated, the balloon is pulled until resistance is felt. On addition of more pressure, the proximal portion is inflated, which fixes the valve in the middle waist portion of the valve. The middle waist has the least compliance and dilates only when substantial pressure is added to the balloon, thereby securing the balloon across the valve prior to the dilation of the annulus.
In our institution, we routinely use TEE to guide the valvuloplasty in order to assess the result of balloon inflation (in addition to simultaneous left atrial [LA]-LV
gradient measurement) and, more importantly, to evaluate the degree of MR. Substantial increases in MR should preclude further inflation. In addition, the procedure should be aborted in the presence of left atrial appendage (LAA) clot. Some institutions have gained facility in using intracardiac echocardiography (ICE) in this application.
gradient measurement) and, more importantly, to evaluate the degree of MR. Substantial increases in MR should preclude further inflation. In addition, the procedure should be aborted in the presence of left atrial appendage (LAA) clot. Some institutions have gained facility in using intracardiac echocardiography (ICE) in this application.
C. Complications.
The most common serious complications include hemopericardium or severe MR. Perforation of cardiac chambers, which occurs with a rate of 0% to 2%, may happen while manipulating the catheters in the heart. While an increase in MR may routinely be noted after the PMBV, it rarely requires a surgical intervention.
D. Outcomes.
Immediate postprocedural success with a final valve area > 1.5 cm2 without moderate or severe MR is the best predictor of long-term outcome. The best results are obtained in young people with favorable anatomic characteristics. Randomized clinical trials have demonstrated that long-term results of PMBV in young patients are as good as open commissurotomy and are better than closed commissurotomy. In patients with optimal morphology, freedom from restenosis has been reported as 92% at 5 years, 85% at 10 years, and 65% at 15 years. Repeat PMBV has been recommended as first-line therapy in patients with symptomatic mitral restenosis after PMBV or commissurotomy in whom the mechanism of restenosis is commissural fusion.
III. BALLOON AORTIC VALVULOPLASTY (BAV)
A. Background.
Degenerative or calcific AS is one of the most common valvular disorders encountered in western countries. Surgical aortic valve replacement (SAVR) is the treatment of choice for patients who are safely able to undergo cardiac surgery, and transcatheter aortic valve replacement (TAVR) is emerging as a favorable option in patients at high risk for surgical complications. Balloon dilation of the calcified aortic valve results in stretching of the fused commissures. Due to rapid reversibility of these effects, there is an early loss of effectiveness in severe degenerative AS, and the valve returns to pre-BAV size in 3 to 6 months.
In patients with severe AS who are hemodynamically unstable and for whom urgent aortic valve replacement (AVR) is not feasible, BAV may serve as a “bridge” to valve replacement. Similarly, we have also seen significant functional improvement in patients after BAV so that patients unable to undergo AVR initially have improved to a point that TAVR or SAVR could be performed safely. In patients who require urgent noncardiac surgery, BAV may be considered as a temporizing measure in the hope of reducing the risks of perioperative hemodynamic changes associated with anesthesia.
A number of patients with severe AS have other comorbidities, such as chronic obstructive pulmonary disease or liver or kidney disease, that make it difficult to discern the degree to which AS contributes to their symptoms. In such cases, BAV may provide a therapeutic answer; improvement of symptoms points to AS as the driver of symptoms and may push for a more definitive valve replacement option. Finally, in patients without any option for either TAVR or SAVR, BAV may be considered as a palliative measure.
B. Procedure.
The femoral retrograde approach is the most commonly utilized method for BAV, although in patients with severe iliofemoral disease the procedure can be performed in an antegrade fashion via venous access and transseptal puncture. A Swan-Ganz catheter is placed in the pulmonary artery for continuous hemodynamic monitoring and assessment of cardiac output. A temporary pacemaker is placed in the right ventricle to perform rapid pacing (180 beats per minute) during balloon inflation to reduce cardiac output and minimize balloon movement in the annulus. After crossing the aortic valve using a 5F AL-1 diagnostic catheter and a straight wire, a stiffer wire is inserted and positioned in the left ventricle. BAV is typically performed using balloons ranging from 15 to 25 mm in diameter. The balloon is sized based on the annulus diameter on transthoracic echocardiography
(TTE); the maximum balloon size is 10% larger than the annulus, and we routinely begin dilation at smaller sizes and assess the hemodynamic result prior to increasing the balloon size. Procedural success with BAV is typically defined as a 50% reduction in mean aortic valve gradient and a 25% increase in aortic valve area (AVA); most patients usually experience almost a 50% increase in AVA
(TTE); the maximum balloon size is 10% larger than the annulus, and we routinely begin dilation at smaller sizes and assess the hemodynamic result prior to increasing the balloon size. Procedural success with BAV is typically defined as a 50% reduction in mean aortic valve gradient and a 25% increase in aortic valve area (AVA); most patients usually experience almost a 50% increase in AVA
C. Complications.
It should be noted that BAV carries considerable risk. The 30-day mortality associated with the procedure may be up to 10%, usually due to either aortic regurgitation (as a complication of the balloon procedure) or persistent heart failure. Other complications (occurring in up to 15%) include stroke, peripheral vascular complications (due to the size of the devices used and concomitant incidence of peripheral arterial disease), coronary occlusion, need for permanent pacemaker implantation, cardiac tamponade, and cardiac arrest.
D. Outcomes.
Despite a modest improvement in valve area, a significant improvement in the functional status is noted after BAV. However, the benefit of BAV gradually disappears over the course of the next few months. The poor functional status of the patients, as well as only moderate, transient effects of the technique, is primarily responsible for the overall grim long-term results of stand-alone BAV Although there are no contemporary studies comparing SAVR with BAV, extensive data indicate that stand-alone BAV does not change the natural course of AS, even after repeated procedures. Despite this, BAV holds an important place in the treatment of patients with severe AS. In our current experience, BAV is most often performed to bridge severely symptomatic patients to TAVR or SAVR or to better understand the contribution of AS to functional limitation in patients with multiple comorbidities. BAV has tremendous potential to alleviate symptoms and provide an opportunity for functional improvement that allows definitive treatment with AVR and improved quality and quantity of life in patients with severe AS.
IV. TRANSCATHETER AORTIC VALVE REPLACEMENT
A. Background.
Up to a third of patients with severe symptomatic AS do not undergo surgical AVR, as they are deemed to have a high surgical risk due to age or multiple comorbidities. The interest in percutaneous aortic valve implantation began in the early 1990s. The first human experience was reported by Cribier et al. in 2002. Since then, rapid advancements in the design of the stented valve and delivery catheters and improved facility in implantation techniques have led to a consistent improvement in the postprocedural outcomes and have heralded a new era in the treatment of valvular AS.
The procedure involves implantation of a tissue pericardial valve that is mounted within a stent. The key to TAVR success is a careful selection of patient population for the procedure and a judicious use of preprocedural and intraprocedural imaging modalities, including fluoroscopy and aortic angiography.
Currently, the indications for TAVR include severe symptomatic AS with a valve area of ≤ 0.8 cm2, mean aortic valve gradient of ≥ 40 mm Hg, or a peak aortic jet velocity of ≥ 4.0 m/s with one or more of the following:
(1) High risk for conventional AVR (Society of Thoracic Surgeons score > 10 or logistic EuroSCORE > 20%) (web site 209.220.160.181/STSWebRiskCalc/).
(2) Contraindication to standard thoracotomy including prior multiple thoracotomies or radiation to the chest wall.
(3) Porcelain aorta.
B. Procedure.
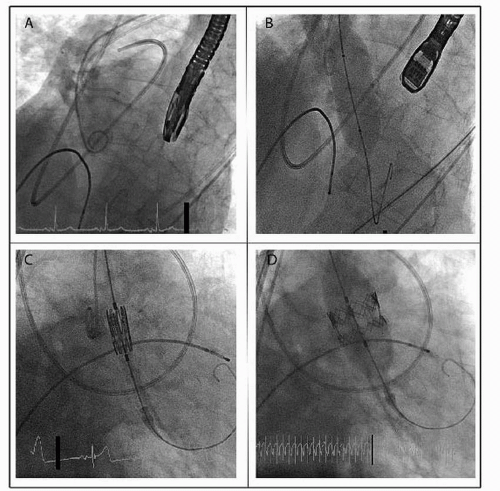
Two types of stented valves have been tested in humans: the balloonexpandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA) and the selfexpanding Core Valve ReValving system (Medtronic Inc., Minneapolis, MN) (Fig. 66.2).
1. Edwards SAPIEN.
The largest human experience is with the Edwards Lifesciences series of balloon-expandable aortic valves. The valve consists of a tubular
stainless steel stent with a fabric valve cuff and contains valve leaflets derived from bovine pericardial tissue. This valve is available in two sizes, 23 mm (via 22F sheath access) and 26 mm (via 24F sheath access), and refers to the fully expanded internal stent diameter. Generally, a 23 mm valve is implanted in annuli that measure 18 to 22 mm, and a 26 mm valve is implanted in annuli that measure 23 to 26 mm. Although the valve was implanted initially using the transvenous access and subsequent transseptal puncture, it is now routinely implanted using either a retrograde transfemoral approach or an antegrade transapical approach. Furthermore, the newest iteration of the valve, the SAPIEN XT, which consists of a cobalt chromium stent and allows a smaller sheath size for insertion (18F for 23 mm valve and 19F for 26 mm valve), is currently in use in the Placement of Aortic Transcatheter Valves (PARTNER) II study.
stainless steel stent with a fabric valve cuff and contains valve leaflets derived from bovine pericardial tissue. This valve is available in two sizes, 23 mm (via 22F sheath access) and 26 mm (via 24F sheath access), and refers to the fully expanded internal stent diameter. Generally, a 23 mm valve is implanted in annuli that measure 18 to 22 mm, and a 26 mm valve is implanted in annuli that measure 23 to 26 mm. Although the valve was implanted initially using the transvenous access and subsequent transseptal puncture, it is now routinely implanted using either a retrograde transfemoral approach or an antegrade transapical approach. Furthermore, the newest iteration of the valve, the SAPIEN XT, which consists of a cobalt chromium stent and allows a smaller sheath size for insertion (18F for 23 mm valve and 19F for 26 mm valve), is currently in use in the Placement of Aortic Transcatheter Valves (PARTNER) II study.
 Get Clinical Tree app for offline access 
|