49 Percutaneous Mitral Valve Repair
 Introduction
Introduction
Chronic MR poses a significant public health burden, with more than three million people in the United States alone suffering from moderate or severe MR.1 Left untreated, chronic MR results in heart failure symptoms, left ventricular cavity dilation and systolic dysfunction, left atrial enlargement, atrial fibrillation (AF), and pulmonary hypertension. Medical therapy may provide some relief from symptoms and is necessary to treat ischemic heart disease and heart failure in patients with MR and these underlying disease states. However, there has been no proven benefit to these treatments with regard to MR itself.2 Therefore, surgical correction with mitral valve repair (MVRe) or replacement (MVR) remains the mainstay of therapy for chronic MR. However, because of the invasive nature of open-heart surgery (OHS) and the frequent presence of comorbidities in this group, up to 50% of patients with severe MR may not be offered surgery.3 This is especially true for older patients and those with impaired left ventricular function. As a result, percutaneous technology is poised to significantly alter the treatment paradigm for chronic MR. Percutaneous MVRe offers the potential benefit of decreased morbidity, improved recovery time, and shorter hospital stays compared with OHS. Current percutaneous options are loosely based on surgical repair techniques, with four primary methods to accomplish a reduction in MR: (1) edge-to-edge leaflet repair, (2) indirect coronary sinus annuloplasty, (3) direct annuloplasty, and (4) septal–lateral annular cinching. Percutaneous MVR is also being developed, although, at this time, it is in its infancy. Overall, the field is very exciting with promising initial studies highlighting the need for a better understanding of patient selection for appropriate management of MR.
 Mitral Valve Disease
Mitral Valve Disease
Mitral Valve Anatomy
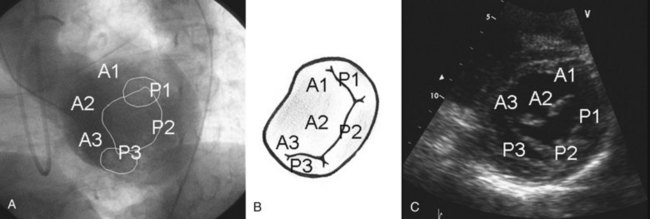
The mitral valve complex is composed of the mitral annulus, anterior and posterior leaflets, chordae tendineae, and papillary muscles.4 The mitral annulus is the elliptical area of attachment of the mitral valve to the base of the left atrium (LA) (Fig. 49-1). The posterior leaflet has three lobes or “scallops”: the lateral (P1), central (P2), and medial scallops (P3); the anterior leaflet segments are named A1, A2, and A3, respectively, corresponding to the posterior scallops. The anatomic position of the valve is such that the two leaflets meet at the anterolateral and posteromedial commissures. Chordae connect the leaflets to both the anterolateral and the posteromedial papillary muscles. The primary chordae connect to the free edge of the leaflet; the secondary chordae (“strut” chords) are thicker and connect to the rough zone of the leaflet; and the tertiary chordae are short and connect the basal zone of the leaflet to the ventricular free wall.
Etiology and Mechanism of Mitral Regurgitation
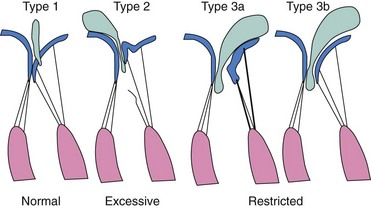
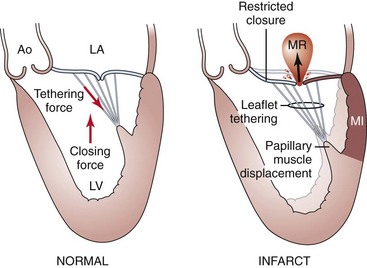
Anatomic or functional abnormalities of any of the structures in the mitral valve apparatus may lead to MR.5,6 The disease process leading to MR may be primary mitral valve disease, secondary regurgitation resulting from another cardiac disease, or mitral valve involvement in a systemic inflammatory disease (Table 49-1). Various terminologies are used to characterize the mechanisms of MR. The morphologic description, proposed by Carpentier, classifies the mechanism of regurgitation according to leaflet pathophysiology (Fig. 49-2).7 Type I regurgitation occurs in the presence of normal leaflet motion and is usually caused by annular dilatation or leaflet perforation. Type II is caused by leaflet prolapse, which is commonly the result of degenerative (myxomatous) disease, chordal elongation or rupture, or papillary muscle elongation or rupture. Type III is caused by restricted leaflet motion, which may arise from posterior wall motion abnormality or papillary muscle dysfunction due to ischemic cardiac disease. The restricted leaflet motion may also be caused by commissural fusion, leaflet or chordal thickening from rheumatic heart disease, or both. This simplification has usefulness in terms of both the surgical approach and the percutaneous approach, as the goal of therapy is to restore normal leaflet function but not necessarily normal valve anatomy. Another common method of categorizing MR is based on the etiology and mechanism of MR. This classification is commonly used in the literature to study the clinical outcomes of patients (Table 49-2). In this classification scheme, MR is loosely categorized on the basis of an abnormal (“primary”) or normal (“secondary”) mitral valve as degenerative or rheumatic disease (primary MR) and functional or ischemic disease (secondary MR), respectively. Of note, the terms functional MR, ischemic MR, and secondary MR are often used interchangeably and may represent many different mechanisms and morphologic variants. Degenerative disease includes Barlow’s disease (myxomatous degeneration) and fibroelastic deficiency, both of which can result in mitral valve leaflet prolapse and MR. Fibroelastic deficiency is the most common etiology in those presenting for surgical MVRe, representing approximately 70% of the surgical population in the United States. MR in rheumatic disease is a result of leaflet deformity caused by severe calcification and apical leaflet doming. Although this is a common cause of MR worldwide, it is less frequently encountered in the United States. Functional MR occurs in the setting of left ventricular dysfunction and is seen in patients with coronary artery disease (ischemic MR) or in patients with dilated cardiomyopathy from other causes. Ischemic MR results from decreased closing force and increased tethering force on the leaflets.8 Various factors can lessen the closing force, including diminished left ventricular ejection fraction (LVEF), left ventricular dyssynchrony, and decreased annular contraction; similarly, many factors increase the tethering force, such as papillary muscle displacement, left ventricle (LV) remodeling, annular dilatation, and so on.9–12 It is becoming increasingly clear that significant interaction among ventricular, valvular, and annular factors is involved in the generation, perpetuation, and progression of “functional” MR (Fig. 49-3).
TABLE 49-1 Causes of Chronic Mitral Regurgitation
TABLE 49-2 Mechanisms and Classification of Mitral Regurgitation
| Carpentier’s morphologic classification with mechanisms of MR |
* Functional mitral regurgitation (MR) sometimes used to describe ischemic and nonischemic MR as both share some common characteristics of left ventricular geometric remodeling and annular dilatation with normal leaflet morphology.
Natural History
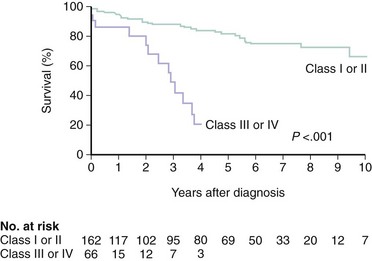
The natural history of patients with chronic MR depends on the degree of regurgitation, the cause of the underlying disorder, and the degree of left ventricular dysfunction.13–15 Available data on the natural history of the disease are limited by small sample size, selection bias, inconsistent measures of MR severity, and the inclusion of disparate etiologies of regurgitation. However, it appears that many patients with chronic MR remain asymptomatic for many years.16 Among patients with mild MR, there is an inconsistent rate of progression to severe MR, which appears to be independent of medical treatment.17 When chronic severe MR is present, approximately 5% to 10% of patients per year develop significant symptoms, clinical indication for surgery, death, or all of these.16,18 Whether there is a small risk of sudden cardiac death (SCD) in patients with severe asymptomatic MR remains controversial, and the data are not compelling enough to subject patients to intervention in the absence of other indications for MVRe.18 The importance of symptoms on long-term prognosis is demonstrated by the high mortality rate reported for patients with New York Heart Association class III or IV symptoms, even in degenerative mitral valve disease (Fig. 49-4). LVEF is also an important independent predictor of outcome in patients with functional MR.15 Patients with degenerative valve disease have a favorable long-term prognosis, whether they are treated conservatively or when indicated, surgically. However, patients with degenerative valve disease and coronary disease are fundamentally different from those with degenerative disease alone and have a worse prognosis that is dominated by the contribution of coronary disease.14 Conversely, in the absence of degenerative disease, the presence and degree of MR after myocardial infarction (MI) is an independent predictor of mortality, emphasizing the need for accurate quantification; further studies to investigate whether treatment of MR in these patients will modify outcomes.19,20
Imaging: Echocardiography
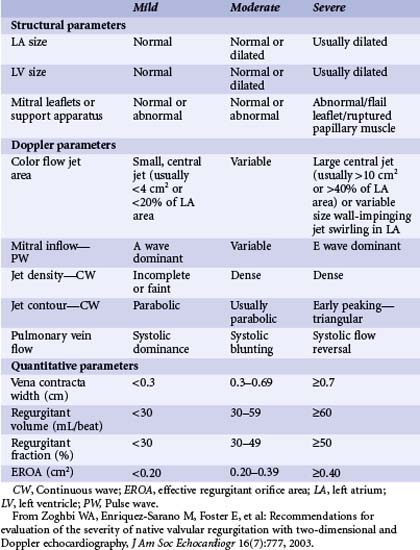
Echocardiography is the dominant modality for imaging the mitral valve and for the assessment of MR severity. Two-dimensional trans-thoracic echocardiography (TTE) is useful to evaluate the valvular anatomy, the structure and function of the LV, and assess the origin and degree of regurgitation. Therefore, TTE provides an understanding of MR severity as well as an insight into the primary or secondary nature of the disease. Furthermore, the anatomic assessment of the mitral valve and the LV also allows determination of whether surgical or percutaneous MVRe may be feasible or whether valve replacement will be necessary. Longitudinal data collected using serial echocardiography may be used to determine the timing of intervention and to follow up the results. If trans-thoracic images are not adequate, trans-esophageal echocardiography (TEE) provides an excellent assessment of mitral valve anatomy and severity of regurgitation. This can be particularly useful to determine if valve repair is feasible. Evaluating the severity of valvular regurgitation with echocardiography relies heavily on Doppler methods, including color, pulsed-wave (PW), and continuous-wave (CW) Doppler. The American Society of Echocardiography has determined the qualitative and quantitative echocardiographic parameters that are useful in grading MR (Table 49-3).21 The assessment of severity should rely on the integration of both quantitative and qualitative measures obtained by Doppler techniques.22 In addition, structural findings such as a flail leaflet or an enlarged left atrium (LA) can add useful information with regard to regurgitation severity. Color Doppler provides a number of qualitative and quantitative means to determine MR severity. A proximal flow convergence on color Doppler is present in severe regurgitation. The proximal isovelocity surface area (PISA) of this flow convergence can be used to accurately quantitate the effective regurgitant orifice (ERO) area.23 The width of the regurgitant jet at or just downstream from the regurgitant orifice is known as the vena contracta and is slightly smaller than the anatomic regurgitant orifice.24 This distinction is particularly relevant in some patients with functional MR, in which the regurgitant orifice has a slit-like rather than oval shape. The area of the MR jet occupying the LA can provide a rapid semi-quantitative assessment of regurgitation severity. However, this is influenced by instrument factors (such as gain) as well as jet orientation (a central jet may appear more severe than an equally large jet that adheres to the atrial wall). In addition, the color jet area is influenced by the driving pressure across the valve and can be enhanced by elevated blood pressure. Color Doppler imaging is also important in the parasternal short-axis view to determine the origin of the MR jet as percutaneous approaches (especially the MitraClip) are more effective in centrally originating jets than in medial or lateral ones. Regurgitant volume can be assessed using continuous-wave Doppler data. Regurgitant volume is calculated by applying the continuity equation (conservation of mass), in which left-sided regurgitation volume is calculated as the difference between Doppler-derived flows across the aortic and mitral valves.25 The stroke volume equals the cross-sectional area of the valve annulus, multiplied by the velocity-time integral of flow across the annulus. The regurgitant volume at the mitral valve is calculated as the difference between stroke volumes across the mitral valve and the aortic valve. This can also be expressed as a regurgitant fraction.
Pulsed-wave Doppler is useful to assess the effect of regurgitation on the pulmonary venous flow. If the pulmonary venous flow is blunted or reversed in systole, this can indicate severe regurgitation.26,27 The contour and density of the regurgitant envelope on continuous-wave Doppler is also useful, as a dense, early-peaking, or triangular envelope is most consistent with severe regurgitation. In addition, the mitral inflow pattern is typically E-wave dominant (>1.2 m per second) in severe regurgitation, reflecting increased flow across the valve.
Alternative Imaging Modalities
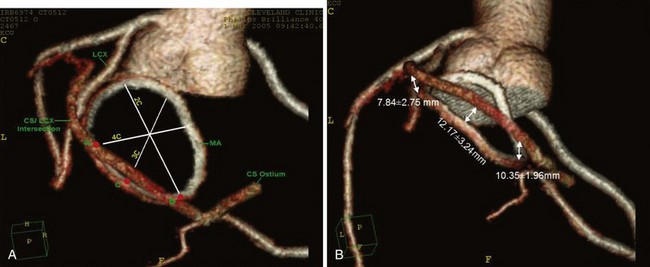
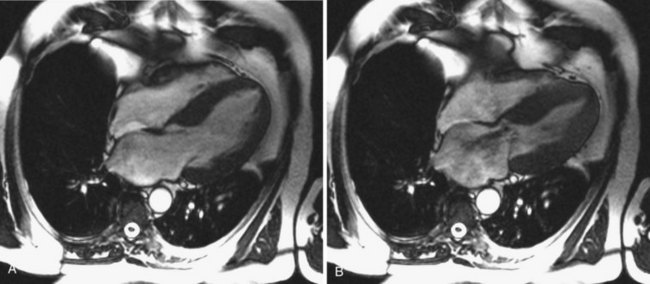
Although historically echocardiography has been the dominant imaging modality in the assessment of MR, cardiac computed tomography (CT), cardiac magnetic resonance imaging (MRI), and three-dimensional echocardiography are beginning to play more important roles.22,28–30 As coronary sinus devices that indirectly alter annular geometry are under development, the relationship of the coronary sinus to the mitral annulus is becoming increasingly important (Fig. 49-5). In addition, the coronary sinus and left circumflex (LCx) arteries are close to each other and may overlap in more than 90% of patients, creating the potential for cinching devices to hinder coronary blood flow.31 Cardiac CT, therefore, has the potential to provide significant anatomic details in the screening of patients and procedural planning. With cardiac MRI, it is possible to obtain significant structural information regarding the geometry of the left ventricle (LV), the mitral annulus and leaflets, and quantitative regurgitant volumes (Fig. 49-6).30 It is likely that the regurgitant volumes calculated using cardiac MRI may be more accurate and operator or reader independent compared with TTE. However, limited experience with this modality currently limits its usefulness for research purposes. Three-dimensional echocardiography is a developing imaging modality that provides a more in-depth understanding of the anatomy of the mitral valve apparatus and the changes of MR. Because the position of the mitral valve apparatus in the LV can be disrupted in all directions, three-dimensional echocardiography is more likely to define the exact mechanism of MR in ischemic disease and to detect multiple jets of MR that may affect the treatment approach.32 Furthermore, with recent advances, the ability to acquire real-time three-dimensional images allows use of the procedure to gauge safety and efficacy during both percutaneous and traditional surgical repairs.
 Surgical Mitral Valve Repair
Surgical Mitral Valve Repair
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines provide a framework for patient selection and timing of mitral valve surgery.33 Any patient with acute severe MR should undergo valve surgery. Valve surgery is recommended for patients with chronic severe MR in the presence of symptoms and for patients without symptoms in the presence of left ventricular systolic dysfunction or left ventricular cavity dilation (end-systolic dimension >40 mm), and results in the preservation of left ventricular function and improved survival.34,35 The onset of AF or the development of significant pulmonary hypertension (pulmonary artery systolic pressure >50 mm Hg at rest or >60 mm Hg with exercise) in an asymptomatic patient with normal left ventricular size and systolic function is a reasonable indication for surgery. Management of asymptomatic patients with severe MR remains controversial, but surgery can be offered at centers where the repair likely (>90% chance) has a low operative risk.36,37 MVRe is the preferred method of surgical management of MR to restore normal leaflet function and annular size.33 When compared with MVR, the major advantages of MVRe are improved survival, preservation of left ventricular function, freedom from anticoagulation, and fewer complications.38,39 Despite the advantages of MVRe, this technique appears to be under-utilized, as less than 50% of the patients undergoing mitral valve surgery currently receive a repair procedure, even though about 90% are suitable.40,41
Surgical Approach
Mitral valve surgery can be performed by using median sternotomy, a minimally invasive approach that uses partial upper sternotomy or small right thoracotomy, or it can be performed robotically through multiple “ports.” Median sternotomy is required if concomitant coronary bypass is undertaken. Cardioplegic arrest and cardiopulmonary bypass are necessary regardless of the type of chest wall incision, although typically less than 1 hour is required for a valve repair.7 Techniques of repair address the annulus (annuloplasty with or without a rigid or flexible ring, decalcification, débridement), the leaflets (triangular or quadrangular resection, sliding annuloplasty, patch enlargement, decalcification, edge-to-edge repair), the chordae (resection or elongation of chords), the myocardium itself (remodeling through the Dor procedure, plication of scar, pericardial cushions, trans-ventricular slings, etc.), the papillary muscle (realignment), or some combination of all these.
Isolated Annuloplasty
Available annuloplasty techniques include suture alone, suture with buttressing material, or prosthetic annuloplasty devices. The choice of annuloplasty technique is still being debated among surgeons. A prosthetic annuloplasty band or ring is placed to correct annular dilatation, increase leaflet coaptation by reducing the anteroposterior dimension of the annulus, and prevent future annular dilatation. Of these, the Cosgrove–Edwards annuloplasty band and the Carpentier–Edwards annuloplasty ring are the most commonly used. The Cosgrove–Edwards device is placed along the posterior annulus, which has been shown to be the area of greatest annular dilatation in degenerative and functional MR. The location and flexibility of this band may allow preservation of normal annular motion, although this has not been demonstrated to influence clinical outcome.42 Functionally, the annuloplasty ring has the effect of transforming the mitral valve into a monocuspid valve, as the posterior leaflet motion is frequently restricted after the repair. More rigid and undersized rings are used in the treatment of functional MR, although the exact type of ring remains controversial. Annuloplasty for functional MR results in significant improvement in NYHA class, decreased hospital admissions for heart failure, and modest survival rates of 71% to 82% at 2 years and 58% at 5 years. Although favorable changes in left ventricular size, shape, and function have been demonstrated after successful mitral valve repair, a propensity analysis failed to demonstrate any mortality benefit compared with matched patients not undergoing valve surgery.37,43 The recurrence rate of functional MR after isolated annuloplasty is disappointing (28% at 1 year) and remains a limitation to widespread use of the procedure.44
Annuloplasty with Leaflet Repair
The combination of annuloplasty and leaflet repair, referred to as Carpentier’s techniques, is most frequently performed for degenerative mitral valve disease. Of the surgical methods of mitral leaflet repair, correction of posterior leaflet or bi-leaflet prolapse is the most common. Posterior leaflet prolapse occurs in the majority of cases of degenerative mitral valve disease and is the primary cause of regurgitation in approximately 50% of patients. Prolapse is a result of chordal elongation or rupture and affects the P2 segment most frequently. This type of problem is most frequently corrected by posterior leaflet quadrangular resection and plication of the valve annulus.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree