Percutaneous Coronary Intervention
Bernhard Meier
Overview
Percutaneous coronary intervention (PCI), previously called percutaneous transluminal coronary angioplasty (PTCA), has continuously gained importance since the first procedure in 1977 to become the most common major medical intervention. It can be performed using local anesthesia, even as an outpatient procedure. For optimal performance, it requires the best current radiographic and dilatation equipment as well as a properly trained operator with an experienced crew.
PCI works best for single-vessel disease, but it may be of great value for double- and triple-vessel disease, particularly in patients with discrete lesions or in those who are old, fragile, or have had prior coronary artery bypass grafting (CABG). PCI plays a dominant role in the treatment of acute coronary syndromes, in particular ST-segment elevation myocardial infarction.
Limitations of PCI are low success rates in old and long chronic total coronary occlusions, fatal outcomes in approximately 1% of cases, acute and late ischemic complications in approximately 3% and 2%, respectively, and clinically significant restenosis within the first months in approximately 10%. The strengths of PCI are a greater than 90% success rate, the possibility for a prompt return to a normal physical life, the repeatability of the procedure, and the paucity of complications after the first day or of recurrences after the first year. Stents have proved an invaluable complement to the balloon. Routine stenting (hence direct stenting) has become the standard approach, although it is unequivocally required in a minority of lesions. Drug-eluting (active) stents are about to supplant bare (passive) stents completely.
Current research is focused on technical advances in the crossing of chronic total coronary occlusions and on optimized antiplatelet and antithrombin therapy to reduce acute occlusions. The restenosis problem, already ameliorated by stenting, has been further diminished by active stents, which continue to be a main line of research and development.
Glossary
Ad hoc
Performed during diagnostic catheterization.
Culprit lesion
Lesion responsible for the current event or predominant symptoms.
Fr
French, diameter unit for equipment; 1 Fr = 0.33 mm.
Guidewire
Wire over which to advance the balloon catheter.
Guiding catheter
Catheter through which to introduce the balloon catheter into the coronary artery.
PCI
Percutaneous coronary intervention.
PTCA
Percutaneous transluminal coronary angioplasty.
History
Coronary balloon angioplasty is an offspring of transluminal angioplasty of peripheral arteries initiated by Dotter and Judkins in 1964 (1). Their method of dilating stenoses by successively introducing coaxial catheters of growing diameters was crude. It required an access hole commensurate with the target lumen.
On February 12, 1974, Gruentzig performed the first balloon angioplasty in a peripheral artery at the University Hospital in Zurich, Switzerland, by using a form-constant polyvinyl chloride balloon (2,3). In 1975, he presented a double-lumen balloon catheter, introduced over a guidewire (2). In 1976, he demonstrated the feasibility of coronary balloon angioplasty in dogs. On March 22, 1976, a first human case had to be aborted before introducing the balloon catheter. The coronary ostium of a patient with inoperable end-stage coronary artery disease could not be engaged with the guiding catheter introduced through the arm because of occluded iliac arteries. On May 9, 1977, a first intraoperative balloon angioplasty procedure was accomplished in San Francisco by the cardiologists Gruentzig and Myler and the cardiac surgeon Hanna.
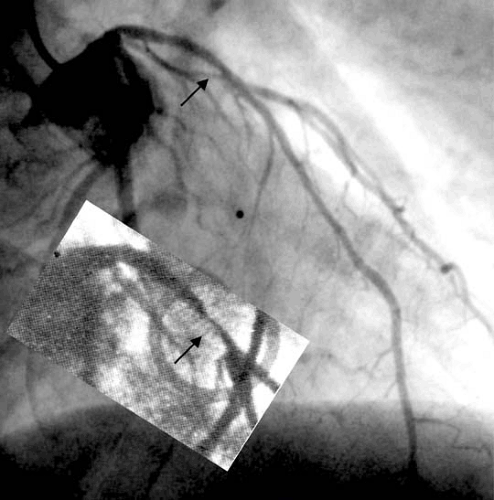
Gruentzig’s historical first PCI case in Zurich of September 16, 1977 concerned a man his own age (38 years) who had a
single discrete stenosis of the left anterior descending coronary artery (4). As the fellow in charge of the patient at the time, I have been partaking in his care ever since. He has remained free of complications and local recurrence (Fig. 78.1) for about 30 years now, a living triumph of the method (5,6). Primary success in the first 50 patients in Zurich was 64% without mortality. Emergency bypass operation was required in 14%, and infarction occurred in 6% (7,8).
single discrete stenosis of the left anterior descending coronary artery (4). As the fellow in charge of the patient at the time, I have been partaking in his care ever since. He has remained free of complications and local recurrence (Fig. 78.1) for about 30 years now, a living triumph of the method (5,6). Primary success in the first 50 patients in Zurich was 64% without mortality. Emergency bypass operation was required in 14%, and infarction occurred in 6% (7,8).
The first clinical use of a coronary stent, on March 28, 1986, by Puel in Toulouse, France (9), induced a gradual change of the pattern of PCI. The stent, first a rare ally of the balloon, matured to become its conjoined twin, in contrast to the balloon’s many, other mostly short-lived, companions or alternatives.
To date, PCI is thriving in spite of a tight medicoeconomic situation. With about 3 million yearly procedures performed worldwide, it tops the list of major medical interventions. In 3 decades, PCI has withstood the scrutiny of local and global peer review. It has been analyzed in numerous registries and subjected to a flurry of well-focused and meticulously monitored randomized studies. It has seen its indications expanded, cropped, and expanded again. It is far from perfect but likable and utterly useful, just as Gruentzig had presaged. He had set out for a humble 15% PCI share of the patients needing coronary revascularization. Up to his accidental death in 1985, he did not envision that PCI was ultimately going to be carried out several times more frequently than CABG.
Mechanism
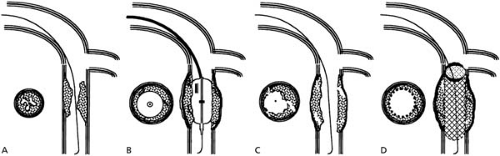
Figure 78.2 explains that dissection of intima and media, usually in thin areas of the plaque or adjacent to it, and dilatation of the vessel circumference constitute the key mechanisms for the luminal gain achieved by PCI (10). The stent prevents elastic recoil and keeps loose intimal flaps or plaque components out of the way. Reendothelialization commonly smoothes the rough surface within a few weeks. Yet it also renarrows the lumen and contributes to restenosis, together with constrictive remodeling in unstented lesions (11).
Procedure
Personnel
Primary Operator
Table 78.1 lists proposed curricula to independence and activity thereafter (12,13,14,15,16). Minimum records are acceptable, but with a history of a more active training period and support by an experienced interventional environment (peers and team). The mortality rates decrease with the patient volume of a center, plateauing at about 600 yearly cases (17). The individual operator volume appears to affect complications less conspicuously, but further improvement beyond 75 cases per year can be demonstrated, particularly in high-risk patients (18).
Assistants
At least one person, using sterile garment, is advisable to assist the primary operator with catheter exchanges and manipulations. An additional person has to be on hand to fetch material, tend to the patient, and summon help in case of need. A single physician suffices in general, but an additional physician with advanced resuscitation skills must be available within minutes and a spare angioplasty operator within the hour. The
unpredictable nature of coronary artery disease in general and of freshly dilated coronary arteries in particular requires on-call 24-hour service.
unpredictable nature of coronary artery disease in general and of freshly dilated coronary arteries in particular requires on-call 24-hour service.
Surgical Standby
A surgical standby under the same roof is ideal (15,19). However, coronary angioplasty without in-house surgery facilities is a valid option to foster ad hoc procedures and to avoid waiting lists. There are important prerequisites for PCI without in-house surgery capability (15). There should be no local legal objections, and the respective operator should be particularly well trained, the case selection adapted, the patient informed, and the institution fully equipped and staffed for advanced resuscitation and intensive care. A disaster plan with transfer options is warranted.
Material
Table 78.2 lists current standard and optional gear for PCI. Aortic counterpulsation is generally considered mandatory, albeit not helpful in hemodynamic collapse. True percutaneous left ventricular support (20,21) and replacement (20) techniques are more intricate but effective. However, the rare need for them makes their availability reasonable in high-volume centers only.
Radiographic Equipment
Catheterization laboratories have to meet high standards to be fit for interventional cardiology. Biplane fluoroscopy is helpful but less important than digital image enhancement and radiation-thrifty flat panel technology. A spare unit providing angulated fluoroscopy should be available for breakdowns during a critical phase of a case.
The storage medium is digital. Exchange is possible via data lines or various recordable disks. Still-frame hard copies are useful for the hospital chart, the patient, and the referring physician.
Monitoring Equipment
Pressure curves and electrocardiographic (ECG) tracings have to be displayed throughout the case. Pertinent ECG changes
and symptoms, such as ST-segment alterations, malignant arrhythmia, and chest pain during balloon inflation, should be documented. A device to measure the activated clotting time is welcome but not indispensable.
and symptoms, such as ST-segment alterations, malignant arrhythmia, and chest pain during balloon inflation, should be documented. A device to measure the activated clotting time is welcome but not indispensable.
TABLE 78.1 Curriculum of Interventional Cardiologists Before and After Independence | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
TABLE 78.2 Equipment and Drugs for Percutaneous Coronary Intervention (in 2005) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Balloon Catheter
A modern balloon catheter comes in a variety of diameters and lengths, is sleek, has a slippery coating inside (minimal guidewire friction) and outside, has a low crossing profile and good “trackability” and “pushability,” inflates and deflates rapidly, and tolerates pressures up to 30 bar with contained expansion (compliance).
Guiding Catheter
The coronary guiding catheters are derived from diagnostic catheters in terms of shapes and materials. Their walls are thinner but still torque true and kink resistant. Diameters of guiding catheters currently range from 5 to 10 Fr (1.7 to 3.3 mm outer diameter).
Guidewire
The guidewire has to transmit torque reliably from the stiff outside end to the floppy tip. It should provide minimal resistance during advancement but optimal support for the catheters introduced over it. A diameter of 0.014 in (0.36 mm) is standard. Hydrophilic coating reduces friction and guarantees easy advancement, but it harbors the risk of occult perforations of thin-walled healthy peripheral coronary arteries with subsequent hemopericardium. The wire tip may be equipped with a Doppler ultrasound crystal, a pressure transducer, a ball tip, a radiofrequency transmitter, or an optical lens for angioscopic tissue analysis or laser transmission.
Accessories
An adjustable Y connector prevents leaking and permits contrast medium injections and pressure monitoring through a single arterial access after the introduction of the guidewire and the balloon catheter into the guiding catheter. A wire torquer ensures controlled rotation of the wire tip. An indeflator with an incorporated pressure gauge affords effortless inflations (in fact, a misnomer for fillings with diluted contrast medium) and maintains constant negative pressure in the balloon lumen of the catheter while the balloon is not inflated.
Technique
The classic point of access is the right femoral artery. Alternatives are the left femoral artery as well as the radial, brachial, and subclavian arteries. Femoral puncture site closure devices (absorbable plugs, clips, or subcutaneous sutures) have shortened bed rest and have facilitated management of the femoral puncture site without significantly reducing complications, however (22). The radial approach features easy hemostasis and immediate mobilization. Yet it is painstaking, limits the selection of catheters and instruments, and carries a risk of radial artery occlusion (23). This condition is generally symptom free initially but may become relevant later (e.g., occlusion of ulnar artery or need for arterial grafts at subsequent CABG). The brachial and axillary routes harbor a significant risk for injury of the motor nerves of the arm.
It is useful to observe ECG and symptomatic reactions of the patient during balloon occlusions. Marked ST-segment elevation, severe pain, malignant ectopy, or a significant decrease in blood pressure mandates close monitoring after the intervention. Collaterals seen during the diagnostic study or documented by washout collaterometry (prompt washout of contrast medium distal to the balloon, injected and secluded there during the initial phase of balloon inflation) (24) project a low subsequent risk for the patient and allow for abridged and simplified aftercare.
Adjunctive Drug Therapy
Platelet inhibitors have to be administered at the latest at the inception of the procedure (16). Acetylsalicylic acid is standard. Clopidogrel, a thienopyridine, is also advocated, preferably starting a few days before the procedure or with a 600 mg or greater oral loading dose (25,26).
Intravenous direct glycoprotein IIb/IIIa receptor blockers (abciximab, tirofiban, or eptifibatide) have proved efficacious conceptually (27) and in large clinical trials (28,29,30). Their effect is more marked in truly acute coronary syndromes
documented by troponin elevation but is not necessarily superior to that of clopidogrel (31).
documented by troponin elevation but is not necessarily superior to that of clopidogrel (31).
Heparin is part of the standard regimen. Doses vary, but they are usually weight adjusted and are assessed by activated clotting times to be increased in lengthy procedures. Low-molecular-weight heparin has been tested in the setting of PCI; it has proved at least as effective as unfractionated heparin, and it causes less propensity to bleeding (32,33). Whether the difference in bleeding also pertains to smaller femoral catheters or to the radial approach remains to be seen.
At least equivalent efficacy and reduced bleeding were documented for the selective factor Xa antagonist fondaparinux, when it was compared with low-molecular-weight heparin in acute coronary syndromes with or without PCI (34). Of the direct thrombin antagonists, bivalirudin has proved equivalent or superior to a combination of unfractionated heparin and a glycoprotein IIb/IIIa receptor blocker in general cohorts (35,36,37), as well as in patients with diabetes (38) or renal failure (39).
Aftercare
The level of surveillance after the procedure depends on the clinical situation and the result of PCI. ECG monitoring for a few hours is advised but not indispensable for the routine case. Any bleeding from the puncture site is carefully assessed. Manual compression and device compression are first-choice measures. The need for duplex ultrasound examination or surgical repair remains exceptional in experienced centers. Chest pain prompts an immediate ECG for comparison with the baseline tracing. Patients with nonabating chest pain are monitored for arrhythmia and are reinvestigated by catheterization.
Hospital discharge of patients with uncomplicated cases usually occurs the morning after the intervention, but outpatient PCI is feasible in selected cases. A control ECG before discharge should be standard, and cardiac enzymes should be checked in case of problems. Performance of a stress test before discharge is rare, albeit safe (40).
Indications
The steady increase of yearly PCI cases is mainly the result of an earlier invasive diagnosis of coronary artery disease in addition to a more aggressive attitude with elderly patients regarding referral for coronary angiography. Many patients undergoing PCI today would have been treated medically 2 decades ago without invasive investigation. Triple-vessel disease involving all major arteries has largely remained the domain of CABG. The share of multivessel PCI in a single session has not increased since 1992 in Europe (41) (Fig. 78.3), despite the use of stents.
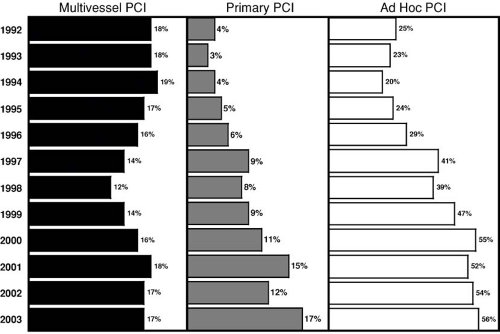 FIGURE 78.3. Percentage of percutaneous coronary intervention (PCI) procedures for more than one major vessel in a session (multivessel PCI, left), for acute myocardial infarction (primary PCI, center), and performed during the diagnostic session (ad hoc PCI, right) in Europe since 1992. Despite the steep increase of stent use during that period, multivessel percutaneous coronary interventions in a single session did not increase. Primary PCI quadrupled and ad hoc PCI doubled during the period. The figures are based on the official registry of the European Society of Cardiology, representing roughly 500,000 procedures per year. Data from reference 41. |
TABLE 78.3 Clinical Indications for and Contraindications to Percutaneous Coronary Intervention | |
|---|---|
|
Clinical Indications
Clinical indications and contraindications are listed in Table 78.3. They have to be customized to patients and situations, and this requires experience. Most patients undergoing coronary angiography meet the criteria. Age is not as important for PCI as it is for CABG. PCI in the elderly is feasible, but its effect is short-lived (42).
Angiographic Indications
Angiographic indications and contraindications are listed in Table 78.4.
Lesion Location and Characteristics
It is important but difficult to determine what makes a lesion significant enough to warrant PCI (43). There are different means to assess the functional significance of a stenosis. Clinical tests (e.g., exercise ECG, stress echocardiography, scintigraphy) are not very sensitive and lack specificity for the individual lesion. Quantitative coronary angiography of the lesion is imprecise almost to the degree of visual estimates (44). Intravascular ultrasound (45), flow velocity (46) or pressure measurements (47), and angioscopy (48) are more objective, but they engender additional costs and risks and have failed to gain widespread acceptance.
To restrict indications to hemodynamically significant lesions may be misguided. The ultimate threat is not the occurrence of angina (affecting only quality of life), but rather the thrombotic occlusion of the vessel, causing infarction and death. Although an individual mild stenosis has a low infarct potential, the hazard is clearly there. Besides, collaterals are rare with mild lesions, a finding that renders their occlusion particularly dangerous. Consequently, restricting PCI to flow-limiting lesions neglects its potential for plaque sealing (i.e., significantly reducing the risk of subsequent thrombotic occlusion thanks to the neoendothelium overgrowing the PCI-inflicted inner wound) (49,50). Such an effect is safely assumed based on the event-free long-term course regularly observed after uncomplicated balloon angioplasty. Restenosis after PCI for mild lesions is rare and is highly unlikely to produce infarction because of the nature of the renarrowing (smooth neoendothelium rather than rupture-prone atherosclerotic plaque). Nonetheless, the hypothesis has not been prospectively validated and may be invalidated by the use of a stent (small but significant risk of late stent thrombosis). Methods to elucidate the vulnerability of a plaque seem called for (Table 78.5) (51,52) but they provide merely a snapshot assessment. A vulnerable plaque means instability and calls for action. A stable plaque is not
particularly meaningful because it may become unstable at any time, perhaps even by the touch of the investigational device. Hence, PCI after such assessment will be the rule, rendering the assessment practically moot. Plaque passivation with statins (53) is a proved and ubiquitously applicable alternative or complement to plaque sealing by balloon angioplasty. The latter is attractive solely as an ad hoc procedure for an angiographically detected mild lesion at a strategically important site (proximal in a large vessel), and it remains clinically unproved (54).
particularly meaningful because it may become unstable at any time, perhaps even by the touch of the investigational device. Hence, PCI after such assessment will be the rule, rendering the assessment practically moot. Plaque passivation with statins (53) is a proved and ubiquitously applicable alternative or complement to plaque sealing by balloon angioplasty. The latter is attractive solely as an ad hoc procedure for an angiographically detected mild lesion at a strategically important site (proximal in a large vessel), and it remains clinically unproved (54).
TABLE 78.4 Angiographic Indications for and Contraindications to Percutaneous Coronary Intervention | |
|---|---|
|
TABLE 78.5 Assessment of Plaque Vulnerability (51
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|
|---|