40 Percutaneous Catheter-based Therapy for Valvular Heart Disease
Pulmonary Valve Stenosis
Pathophysiology
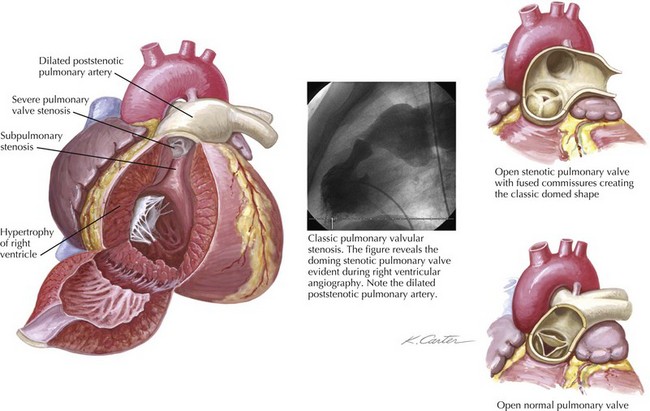
Pulmonary valve stenosis results from fusion of the valve cusps during mid- to late fetal development. The most common form of isolated right ventricular (RV) obstruction, pulmonary valvular stenosis, occurs in approximately 7% of individuals with congenital heart disease (see also Chapters 50 and 52). Pulmonary valve stenosis may be associated with significant RV hypertrophy and infundibular narrowing. The fusion of the valvular cusps produces a classic systolic “doming” appearance angiographically (Fig. 40-1). Tissue pads within the valve sinuses may exist and result in a thickened, rigid valve that is considered dysplastic (a common finding in Noonan’s syndrome). Excessive thickening in dysplastic valves renders the valve unsuitable for percutaneous valvuloplasty, although attempts have occasionally been successful. Similarly, narrowing of the RV outflow tract limits the efficacy of percutaneous balloon techniques. Acquired forms of stenosis are rare (i.e., carcinoid).
Percutaneous Balloon Pulmonary Valvuloplasty
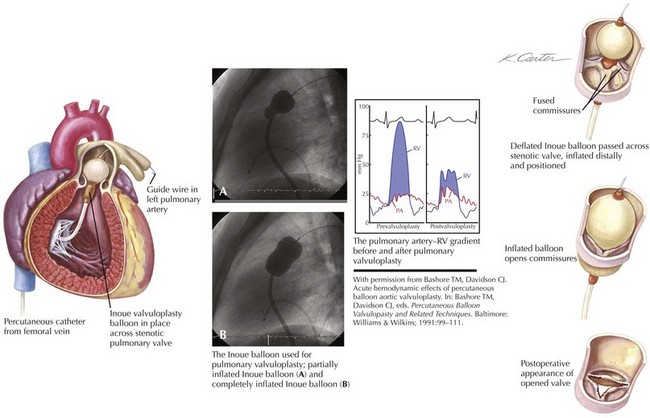
Figure 40-2 demonstrates the gradient between the right ventricle and the pulmonary artery (PA) before and after successful percutaneous balloon valvuloplasty. The RV outflow tract may have considerable muscular subpulmonic stenosis, which may be masked when valvular obstruction is present. The sudden removal of the valvular stenosis after the procedure may result in acute decompensation from marked RV infundibular obstruction, sometimes called the “suicide RV.” Fluid loading, calcium channel blockers, and β-blockers can be used for emergent treatment. After pulmonary valvuloplasty, the subpulmonic hypertrophy may regress considerably over the next several months.
Technique
The dilating balloon or balloons are percutaneously inserted into the femoral vein without a sheath. The maximum inflation of the balloon(s) should be equal to 1.2 to 1.4 times the estimated annular size (see Fig. 40-2). In contrast to the aortic valve (see “Aortic Valve Stenosis”), the pulmonic valve is elastic and often requires oversizing for adequate results. The goal of the procedure is a final peak-to-peak valvular gradient less than 30 mm Hg by cardiac catheterization. Recurrence rates are much lower if that threshold is reached. A single balloon, often 23 mm in diameter in adults, may be used, although two balloons side by side may be necessary in patients with a large annulus. In some laboratories, trefoil or bifoil balloon catheters are available and preferred. The Inoue mitral valvuloplasty balloon (Toray Industries, Inc., Tokyo, Japan) has increasingly been used for pulmonary valvuloplasty because of its stability during inflation.
Aortic Valve Stenosis
Pathophysiology
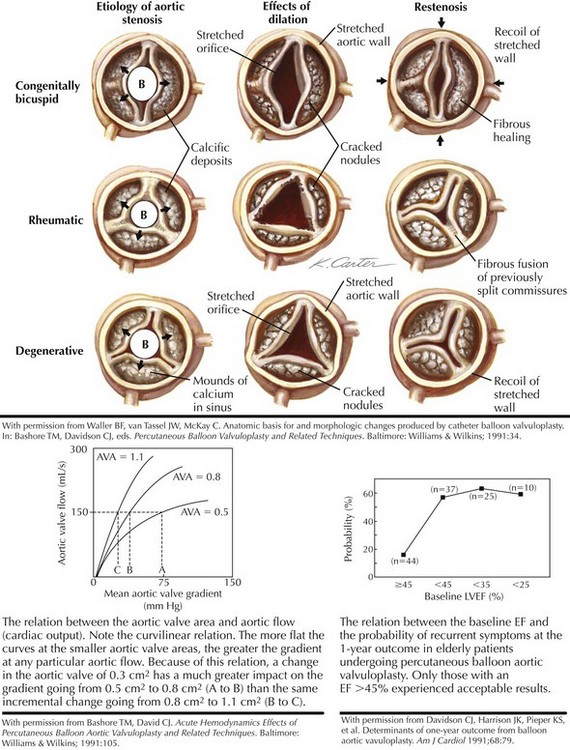
Aortic valve stenosis in elderly persons generally involves a trileaflet valve and probably represents a continuum, from benign aortic valvular sclerosis to severe aortic valvular stenosis. The prevalence of aortic valve sclerosis has been reported to be 25% in individuals older than 65 years of age, with severe aortic valve stenosis evident in 1% to 2% of the population. There is growing evidence that the mechanism of calcific aortic valve stenosis in the elderly is related to atherosclerosis and not to what has commonly been referred to as a “degenerative” process. Little commissural fusion exists; large accretions of calcium can be present in the sinuses of Valsalva. The leaflets gradually lose their flexibility as a result of these calcium deposits. In calcific aortic valve stenosis, the minimal reduction in the gradient that can be obtained by balloon procedures has generally been attributed to cracks in the calcific nodules, cuspal tears, and aortic wall expansion (Fig. 40-3).
When left ventricular (LV) outflow is obstructed at the valvular level, a gradient develops between the left ventricle and the aorta (see Fig. 40-3). The relationship between the gradient and the aortic valve area (AVA) is complex, however, and depends on the severity of the lesion as measured by the AVA and on the cardiac output or the aortic flow. After aortic valvuloplasty, aortic flow may increase because of an improvement in the cardiac output or the development of aortic regurgitation. Either result could increase the gradient, even if the actual AVA also increases. Alternatively, the cardiac output may fall, and the gradient may appear lower even if the AVA has increased. Thus, the short-term postprocedural valvular gradient change may not always reflect the actual change in the AVA.
Technique of Percutaneous Balloon Aortic Valvuloplasty
In contrast to pulmonary valvuloplasty, the balloon catheter used for aortic valvuloplasty should have a maximum inflated diameter slightly smaller than the measured size of the aortic annulus. In adults, a 20-mm-diameter balloon is usually used, although a 23-mm balloon may be required for larger patients. Brief, rapid RV pacing during positioning and inflation of the aortic balloon across the stenotic valve lowers cardiac output transiently and allows for more stable positioning. The balloon catheter is placed in the middle of the valve plane and manually inflated, using dilute (25%) radiographic contrast in saline (Fig. 40-4). Inflation pressures do not seem to influence the outcome significantly, and these pressures are no longer measured. Usually one to three separate 15- to 20-second inflations are adequate.
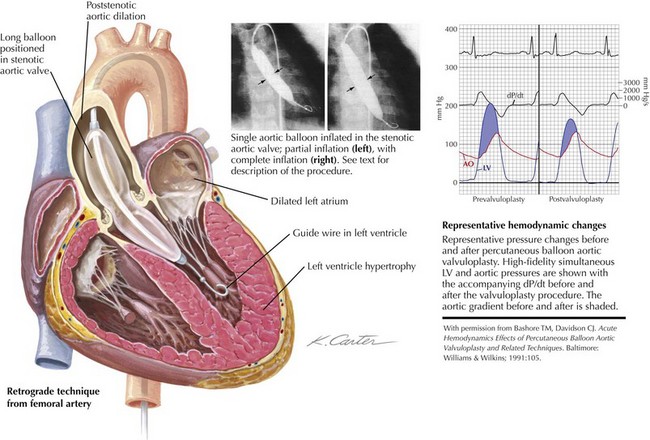
Figure 40-4 Aortic balloon valvuloplasty.
Ao, aortic; dP/dt, delta pressure/delta time; LV, left ventricular.