Percutaneous Cardiac Procedures
Samir R. Kapadia
E. Murat Tuzcu
Overview
Interventional cardiology is evolving rapidly not only to treat coronary disease but also to provide treatment options for various structural heart diseases. These include percutaneous treatment of the valvular heart diseases, atrial appendage occlusion, and treatment of congenital heart diseases. Interventional cardiologists whose patients are adults are involved with increasing frequency in treating atrial septal defect, patent foramen ovale (PFO), and ventricular septal defects.
Many exciting developments in recent years have opened up novel percutaneous avenues to treat valvular heart disease. Balloon dilatation to treat valvular stenosis has been used for many years and has been proven effective for the treatment of pulmonary stenosis (1), mitral stenosis (2), and some cases of aortic stenosis (AS) (3,4). Valvuloplasty is covered in Chapter 82. Until recently, the major limitations of percutaneous valve treatments were the lack of sustained benefit of aortic valvuloplasty and the inability to treat valvular regurgitation. In the last decade, numerous percutaneous devices have been developed for valvular heart disease through close collaboration of interventional cardiologists, cardiothoracic surgeons, and imaging specialists. Percutaneous valve prostheses have been designed for AS and pulmonary regurgitation. Many ingenious approaches that have been engineered for percutaneous repair of mitral regurgitation (MR) are under investigation. These developments have generated a phenomenal amount of interest, both in the scientific community and in the general public. Given the tremendous potential of these techniques in eliminating the need for conventional cardiothoracic surgery and its associated risks—particularly in high-risk patients—and in the treatment of patients who are currently not surgical candidates, the excitement and enthusiasm generated are understandable. In this chapter, we review the devices and approaches that are being investigated for valve treatment, PFO closure, and atrial appendage occlusion.
Aortic Valve Replacement
Scope of the Problem
AS is currently the most common acquired valvular disease in the industrialized nations. With the decline in rheumatic valvular disease, calcific bicuspid and senile degenerative calcific tricuspid valve diseases have emerged as the dominant causes of AS (5). The consequence of prolonged life expectancy in Western countries is a substantial increase in the number of patients surviving to develop senile calcific AS. For adult patients with severe AS, aortic valve replacement (AVR) has been the therapy of choice for more than 3 decades. Such therapy offers dramatic symptomatic relief and improved long-term survival when compared with medical therapy alone (6,7,8). Even though patients undergoing AVR are progressively older and less fit, clinical outcomes following AVR using contemporary surgical techniques and bioprostheses are generally good, with perioperative mortality rates of 2% to 7% (7,9,10,11). However, high-risk subgroups have been identified, including patients with associated coronary artery disease, higher New York Heart Association functional classifications, impaired left ventricular systolic dysfunction, and advanced age, as well as those undergoing emergency surgery. Based on the presence of one or more
of these high-risk features, a significant subset of patients with severe AS is deemed ineligible for AVR (12,13,14).
of these high-risk features, a significant subset of patients with severe AS is deemed ineligible for AVR (12,13,14).
Initial Experiments
In 1986, separate groups in France (15) and the United States (16) reported the feasibility of percutaneous balloon aortic valvuloplasty for the treatment of high-risk patients with severe AS. Valvuloplasty improves the valve area by multiple mechanisms including the fracture of valvular nodular calcification, separation of fused commissures, and plastic deformation of the rigid valve cusps (16,17,18). Although short-term hemodynamic results are acceptable, albeit modest, long-term clinical follow-up following percutaneous balloon aortic valvuloplasty remains disappointing, with near 100% restenosis of the aortic valve at 2 years (3,4,19,20). For this reason, the procedure is now largely restricted to use as a temporizing bridge to surgical AVR and rarely as a palliative treatment for patients with severe symptomatic AS in whom surgery is contraindicated.
The potential for percutaneous AVR was first realized in 1989, when Andersen and colleagues in Aarhus, Denmark, constructed a prosthetic aortic valve that could be successfully implanted in vivo using a transluminal catheter technique (21). The valve was constructed by suturing a dissected porcine aortic valve to a rudimentary stainless steel stent. This stent-valve was then compressed onto a carrier balloon catheter. Because the intention was to seek a percutaneous treatment for aortic regurgitation, these investigators tested the effect of placing the prostheses in both the native subcoronary position and the supracoronary position (i.e., ascending aorta), given that successful positioning in either location would be effective for the treatment of aortic regurgitation. In the initial animal (pig) studies, the large profile of the valve prosthesis necessitated delivery through the suprarenal aorta. The valve was then passed in retrograde fashion to the ascending aorta (i.e., supracoronary location) and aortic valve (subcoronary location) and was deployed by inflation and subsequent deflation of the carrier balloon catheter. Although the valves were successfully deployed, a major issue with the subcoronary position was the propensity of the prosthesis to occlude the origin of the coronary arteries. Compounding this issue was the to-and-fro movement of the carrier balloon during inflation, which made precise positioning of the stent-valve difficult.
Pavcnik and colleagues demonstrated in 1992 that a percutaneous transcatheter placement of an artificial caged-ball valve in animals is feasible, whereas Maozami and coworkers showed in 1996 that a hemodynamically acceptable prosthetic aortic valve for transluminal placement is feasible in an open chest model. Bonhoeffer and coworkers in 2000 successfully performed a percutaneous pulmonary valve implantation in five sheep. Although implantation in the descending aorta was successful, placement in the native position failed because of coronary obstruction, interference with the mitral valve, or migration. These problems were resolved with an orientation device. Sheep anatomy is such that the distance between the mitral valve and the coronary ostia is only a few millimeters, an important limitation of this model.
Cribier-Edwards Aortic Percutaneous Heart Valve (Edwards Lifesciences, Irvine, CA)
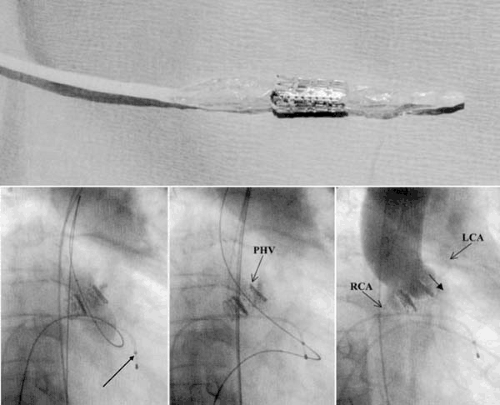
Building on these fundamental experiments, Cribier and his colleagues in Rouen, France, developed a percutaneous heart valve (PHV, Percutaneous Valve Technologies, Inc., Fort Lee, NJ) for the treatment of AS. Following animal testing in sheep (22), and ex vivo valve durability testing, the first human implantation of their PHV was performed in April 2002 and published in December 2002 (23). The initial valve was composed of three leaflets of bovine pericardium sutured to a 14-mm long stainless steel balloon expandable stent. This stent length was specifically chosen based on postmortem assessments in human subjects of the distance between the aortic annulus and both the coronary ostia and anterior mitral leaflet, which could both be impinged by the prosthesis. The stent-valve was mounted on a 30-mm long balloon valvuloplasty catheter (Z-Med II, NuMED, Inc., Hopkinton, NY) that could expand to a diameter of 23 mm. A crimping tool was used to compress the stent-valve symmetrically onto the balloon delivery catheter.
The critical elements of the technique employed by Cribier and his colleagues were as follows (Fig. 79.1) (24). The compressed stent-valve/balloon catheter assembly required a 24-Fr (8-mm) sheath for delivery. For this reason, and to facilitate accurate positioning of the PHV, Cribier and colleagues initially used an antegrade approach for delivery of the PHV. Using femoral venous access, a transseptal puncture was performed, and an 8-Fr Mullins sheath was positioned in the left atrium. A balloon-tipped catheter was then passed through the Mullins sheath and advanced across the mitral valve into the left ventricular outflow tract. Through the lumen of the balloon-tipped catheter, a straight wire was then used to cross the stenosed aortic valve. The deflated balloon-tipped catheter was then advanced across the aortic valve, reinflated, and passed into the descending aorta. A 360-cm long stiff wire was then advanced through the balloon-tipped catheter, grasped by a snare introduced from the femoral artery, and subsequently externalized. Delivery of the PHV was facilitated by dilating the interatrial septum and subsequently dilating the aortic valve with a valvuloplasty balloon (using an antegrade approach). The PHV-balloon assembly was then introduced through the venous access across the interatrial septum and the mitral valve and was finally positioned at the native aortic valve using valvular calcification as a marker. At this stage of the procedure, it was imperative to maintain a large loop of wire in the left ventricle between the mitral and aortic valves, because traction on the anterior mitral leaflet could result in severe MR and hemodynamic collapse. Rapid maximal inflation, deflation, and removal of the balloon were then performed to deploy the valvular prosthesis.
Subsequent to this first reported case description, the procedural and clinical outcomes of this patient and five others were reported in February 2004 (25). Equine pericardial valves were used in the PHV of these subsequent procedures. Some modifications to the original technique were also noted. For example, rapid right ventricular cardiac pacing (200 to 220 beats per minute) was used to decrease aortic blood flow at the time of PHV deployment, thus reducing the risk of migration of the prosthesis into the ascending aorta. In addition, the operators advanced a Sones catheter from the femoral artery access site that abutted the distal end of the delivery balloon catheter, a technique that further limited the tendency for anterograde migration of the PHV during deployment. The device was successfully deployed in five of the six patients. In the unsuccessful case, the PHV-balloon assembly migrated into the ascending aorta at the time of balloon inflation, resulting in hemodynamic collapse and death during the procedure. Among the successful procedures, the hemodynamic results were impressive, with almost complete elimination of the aortic gradient and achievement of an aortic valve area larger than 1.6 cm2. Aortic regurgitation increased by one grade in four of the five successful procedures. In such cases, the aortic regurgitation was paravalvular in location (i.e., between the stent frame of the PHV and the native diseased aortic valve). Importantly, the coronary ostia were not compromised by the prosthesis in any of these patients.
The longer-term clinical follow-up in this series must be interpreted with an understanding of the patient population studied. All patients had severe AS with multiple comorbidities and were deemed ineligible for surgical AVR by two independent surgeons. Most were elderly (mean age, 75±12 years), and three were in cardiogenic shock. Three of the five patients who underwent successful PHV implantation died of noncardiac complications at 2, 4, and 18 weeks, whereas the remaining two patients were alive and free of heart failure at 8-week follow-up. In follow-up echocardiographic studies, the initial reduction in aortic gradient and the increase in aortic valve area were maintained, and there was a significant recovery in left ventricular systolic function.
Based on this preliminary series and on subsequent unpublished reports, the future of percutaneous AVR for the treatment of AS appears promising. The issue of obstruction of the coronary ostia appears to be less of a concern than originally envisioned. However, PHV migration at the time of deployment is a major concern. Additionally, the anterograde approach is technically very challenging and could limit the broader application of the procedure. Cribier reported using the simpler retrograde approach in about 20% of the 40 patients who have now undergone PHV implantation at the Charles Nicolle Hospital of Rouen (as of September 2005). As the profile of the PHV-delivery balloon assembly decreases, the proportion of cases in which this technically less challenging approach may be possible will likely increase. The difficulty of precise positioning of the PHV using the retrograde approach will likely be overcome by certain design modifications. It appears that the increased size of the device to 26 mm has significantly lowered the paravalvular leak problem.
CoreValve (Paris, France; Irvine, CA)
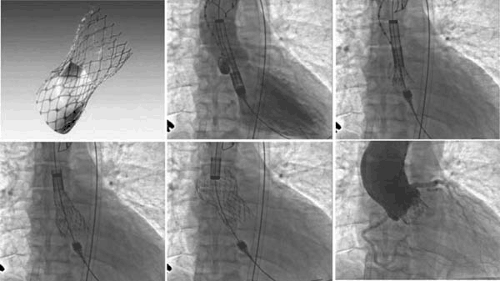
The CoreValve (Revalving) aortic valve consists of a bioprosthetic valve made of bovine pericardial tissue that is mounted and sutured in a self-expanding nitinol stent (Fig. 79.2). The
prosthetic frame (stent) is manufactured by the laser cutting of a nitinol metal tube with a length of 50 mm. The lower part has a high radial force to push aside the calcified leaflets and avoid recoil; the middle part is constrained to avoid coronaries and carries the valve, whereas the upper part expands for fixation in the ascending aorta. The actual valve inner diameter is 21 to 22 mm. For stent deployment, the retrograde approach was used via a surgically prepared common iliac artery as the access site. The procedure was initially performed using general anesthesia with transesophageal echocardiographic (TEE) guidance and femorofemoral cardiac support. Initial results with this valve were reported from 12 patients in whom the 25-Fr system was used. The valve was successfully deployed within the diseased native aortic valve in 10 of 12 patients. One patient died of ventricular rupture before device implantation, and one patient was converted to surgical treatment because of unsuccessful device placement. During in-hospital follow-up, five patients died. Four deaths were procedure related: myocardial perforation (one), crush-syndrome (one), and multiorgan failure (two). Another patient died of lung cancer. All patients developed temporary thrombocytopenia that was persistent if antiplatelet medication was not given. The valve is now in the third generation; the delivery catheter size has been reduced to 21 Fr, and the valve has been delivered using local anesthesia via femoral artery access with a shorter procedural time.
prosthetic frame (stent) is manufactured by the laser cutting of a nitinol metal tube with a length of 50 mm. The lower part has a high radial force to push aside the calcified leaflets and avoid recoil; the middle part is constrained to avoid coronaries and carries the valve, whereas the upper part expands for fixation in the ascending aorta. The actual valve inner diameter is 21 to 22 mm. For stent deployment, the retrograde approach was used via a surgically prepared common iliac artery as the access site. The procedure was initially performed using general anesthesia with transesophageal echocardiographic (TEE) guidance and femorofemoral cardiac support. Initial results with this valve were reported from 12 patients in whom the 25-Fr system was used. The valve was successfully deployed within the diseased native aortic valve in 10 of 12 patients. One patient died of ventricular rupture before device implantation, and one patient was converted to surgical treatment because of unsuccessful device placement. During in-hospital follow-up, five patients died. Four deaths were procedure related: myocardial perforation (one), crush-syndrome (one), and multiorgan failure (two). Another patient died of lung cancer. All patients developed temporary thrombocytopenia that was persistent if antiplatelet medication was not given. The valve is now in the third generation; the delivery catheter size has been reduced to 21 Fr, and the valve has been delivered using local anesthesia via femoral artery access with a shorter procedural time.
Corazón (Menlo Park, CA)
Corazón’s in situ aortic valve treatment device (Beating Heart Aortic Valve Repair [BHAVR] Demineralization System) allows for beating heart demineralization of the aortic valve to improve native valve function in moderate AS. It may also be used to aid easy implantation of a prosthetic valve with good apposition to prevent paravalvular leak. The system first isolates the aortic valve by placing a catheter into the left ventricle. This catheter has a balloon for occluding the left ventricular outflow tract below the AV. The catheter has an expandable central lumen with temporary aortic valve, thus enabling beating heart aortic valve treatment. The aortic valve is then isolated by the three foam elements molded to fit the aortic sinuses. A small pulsatile balloon proximal to this apparatus provides gentle agitation of the foam elements and AV leaflets. A low-pH solution is used for aortic mineralization. The first clinical use of Corazón’s BHAVR device was on a more severely diseased aortic valve before bioprosthetic replacement, in August of 2005. No clinical adverse events were noted during the Corazón treatment.
Multiple other companies are working on the question of developing technologies to make percutaneous valve replacement a reality. Some of these include Cook, Direct Flow Medical, Edwards Lifesciences, Heart Leaflet Technologies, Medtronic, Palmaz/Baley’s, Sadra Medical, Shelhigh, 3F Therapeutics, and ValveXchange.
Pulmonary Valve Replacement
Scope of the Problem
Most pulmonary valve disease in adult patients represents untreated congenital pulmonary stenosis or pulmonary regurgitation (with or without stenosis) following reconstruction of the right ventricular outflow tract (RVOT) in patients with tetralogy of Fallot, pulmonary atresia, or transposition of the great arteries (26). These reconstructions may leave the native
RVOT intact and may include one or more of the following procedures: resection of infundibular tissue, pulmonary commissurotomy, and placement of a patch in the outflow tract, annulus, or pulmonary artery. With such repairs, the natural history is for the development of pulmonary regurgitation, which is severe in more than one third of patients (27). Alternatively, the native RVOT may be bypassed using a valved or valveless extracardiac conduit between the right ventricle and the pulmonary artery (28,29,30). Pulmonary regurgitation is immediate with valveless conduits. Valved conduits are associated with progressive degeneration of the conduit and valve resulting in both pulmonary regurgitation and stenosis, such that multiple surgical procedures are typically required over the lifetime of the individual. Pulmonary regurgitation produces chronic right-sided volume overload, right ventricular dilatation, and impairment of systolic and diastolic function (27,31). Clinically, these anatomic complications are manifest by heart failure, atrial and ventricular arrhythmias, and an increased risk of sudden cardiac death. Pulmonary stenosis results in right ventricular hypertension and predisposes the patient to arrhythmias.
RVOT intact and may include one or more of the following procedures: resection of infundibular tissue, pulmonary commissurotomy, and placement of a patch in the outflow tract, annulus, or pulmonary artery. With such repairs, the natural history is for the development of pulmonary regurgitation, which is severe in more than one third of patients (27). Alternatively, the native RVOT may be bypassed using a valved or valveless extracardiac conduit between the right ventricle and the pulmonary artery (28,29,30). Pulmonary regurgitation is immediate with valveless conduits. Valved conduits are associated with progressive degeneration of the conduit and valve resulting in both pulmonary regurgitation and stenosis, such that multiple surgical procedures are typically required over the lifetime of the individual. Pulmonary regurgitation produces chronic right-sided volume overload, right ventricular dilatation, and impairment of systolic and diastolic function (27,31). Clinically, these anatomic complications are manifest by heart failure, atrial and ventricular arrhythmias, and an increased risk of sudden cardiac death. Pulmonary stenosis results in right ventricular hypertension and predisposes the patient to arrhythmias.
Pulmonary valvuloplasty is well established as the primary treatment for adults with congenital pulmonary valve stenosis. The procedure is well tolerated and effective, and it provides a durable benefit (1). Percutaneous stenting of conduit stenosis has also been successfully practiced for several years (32). In contrast, until recently, the only therapeutic option for patients with severe pulmonary regurgitation was surgical valve replacement. The morbidity and mortality associated with operative replacement resulted in a tendency to perform the procedure relatively late, at a time when the degree of reversibility in right ventricular dilatation and dysfunction is likely diminished. In addition, cardiopulmonary bypass may worsen right ventricular function, thereby attenuating the potential benefit of valve replacement. A percutaneous option for valve replacement may help these patients significantly.
Devices and Initial Experience
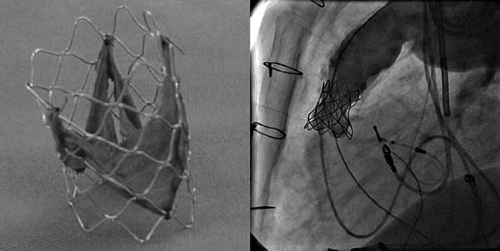
Since 2000, Bonhoeffer and colleagues have championed the development of a percutaneous valve replacement in right ventricle to pulmonary artery prosthetic conduits and more recently in the native pulmonary position (33,34,35,36,37). In design, the valve consists of a piece of bovine internal jugular vein containing a bicuspid or trileaflet valve that is sutured to the interior of an 18-mm diameter balloon expandable stent composed of a soft and highly malleable alloy of platinum-iridium alloy (NuMed, Inc.). Depending on the size of the pulmonary artery or extracardiac conduit, the valve-stent is then hand-crimped onto an 18-, 20-, or 22-mm balloon catheter. With the patient under general anesthesia, and using femoral venous access, a stiff guidewire is placed in the RVOT extending into a distal pulmonary artery branch. The valve-stent balloon assembly is front-loaded into an 18- to 20-Fr (∼6-mm) sheath (NuMed, Inc.) and is delivered over the guidewire to the desired location. Deployment of the prosthesis is achieved by inflation and deflation of the delivery balloon (Fig. 79.3).
Bonhoeffer and colleagues have recently reported their clinical experience with 59 consecutive patients undergoing PV replacement (38). Most of these patients had a variant of tetralogy of Fallot (n = 36), transposition of the great arteries, or ventricular septal defect with pulmonary stenosis (n = 8). The procedure was successful in 58 patients with no mortality. There was subjective and objective improvement in symptoms and exercise tolerance.
Important challenges remain in the broader application of this technology. In patients who have undergone repair of tetralogy of Fallot that maintained the native RVOT by valvotomy, valvectomy, or patch repair, there is often no secure implantation point at the site of the pulmonary annulus. This fact significantly affects the risk of immediate or delayed migration of the device. For patients with extracardiac conduits, variation in conduit size is also a limiting factor. For a conduit
smaller than 16 mm in diameter, there is a danger that the valve-stent assembly will be underexpanded, thus resulting in a larger profile, which may cause obstruction. Conversely, the small size of currently available bovine jugular venous valves (<22 mm) limits the use of this prosthesis for patients with a conduit exceeding this diameter because of the risk of incompetence and/or migration of the valve. Boudjemline, working with the Bonhoeffer group, described a novel pulmonary prosthesis specifically designed for patients with an enlarged conduit or RVOT (37). This device is a self-expandable nitinol stent that has a spontaneous diameter of 30 mm at the ends and a 15-mm-long central restricted area that currently has a diameter of 18 mm. The outer ends ensure fixation of the stent to the wall of an enlarged RVOT or conduit, whereas the central restricted area provides the supporting structure for the 18-mm bovine internal jugular vein valve. A polytetrafluoroethylene membrane is sutured to the outer surface of the nitinol stent to create a seal between the stent and the pulmonary artery. Animal studies with this stent have produced encouraging results that will likely form the basis for human application in the near future.
smaller than 16 mm in diameter, there is a danger that the valve-stent assembly will be underexpanded, thus resulting in a larger profile, which may cause obstruction. Conversely, the small size of currently available bovine jugular venous valves (<22 mm) limits the use of this prosthesis for patients with a conduit exceeding this diameter because of the risk of incompetence and/or migration of the valve. Boudjemline, working with the Bonhoeffer group, described a novel pulmonary prosthesis specifically designed for patients with an enlarged conduit or RVOT (37). This device is a self-expandable nitinol stent that has a spontaneous diameter of 30 mm at the ends and a 15-mm-long central restricted area that currently has a diameter of 18 mm. The outer ends ensure fixation of the stent to the wall of an enlarged RVOT or conduit, whereas the central restricted area provides the supporting structure for the 18-mm bovine internal jugular vein valve. A polytetrafluoroethylene membrane is sutured to the outer surface of the nitinol stent to create a seal between the stent and the pulmonary artery. Animal studies with this stent have produced encouraging results that will likely form the basis for human application in the near future.
Complications from this initial experience can be classified into three categories. The first were related to balloon dilatation or stenting for conduit stenosis, including dissection, hemorrhage from conduit rupture, and residual stenosis because of external compression or undilatable conduits. The second category included issues of patient selection in which dislodgment or embolization of the valved stent occurred. In the third category, complications were thought to be related to device design, which included the “hammock” effect and stent fracture. The hammock effect was resolved with an improved design, whereby the entire length of the stent was sutured to the venous wall segment. Although stent fracture was seen in six patients, only two had clinical consequences. One was treated with another percutaneous valve replacement, whereas the second patient required surgery.
Mitral Valve Repair and Replacement
Scope of the Problem
With the aging of the population and the declining incidence of rheumatic diseases, MR has emerged as the dominant form of mitral valve disease (39). Surgical options for severe MR include mitral valve replacement, with or without preservation of the subvalvular apparatus, and mitral valve repair. The general consensus is that, when feasible, mitral valve repair is superior to surgical replacement because it is associated with lower operative mortality, reduced rates of endocarditis, fewer thromboembolic events, and improved long-term survival (40). The survival benefits of valve repair are likely mediated by preservation of left ventricular function as a result of maintenance of the continuity between the mitral valve and its supporting structures (annulus, chordae tendineae, papillary muscles) (41). However, currently in the United States, mitral valve replacement, not repair, is performed in almost 50% patients undergoing mitral valve surgery.
Relevant Surgical Information
Various surgical techniques for the repair of acquired MR have been developed over the last 3 decades. An appreciation of the underlying mechanism of MR is of paramount importance in the decision regarding the particular surgical technique employed. This task is complicated by the finding that MR is almost always caused by multiple lesions affecting the various components of the mitral apparatus. Despite this complexity, various classification systems of MR have been proposed that serve to highlight the dominant mechanisms involved and to guide appropriate surgical therapies. An understanding of these mechanisms and of the fundamentals of surgical techniques for mitral valve repair is a necessary preamble to any discussion of the novel percutaneous approaches to mitral valve repair.
Carpentier and colleagues proposed three broad categories for the classification of the mechanism of MR (42). This classification has been embraced by the surgical community and is widely used in cardiothoracic literature. Table 79.1 summarizes the characteristic findings, underlying pathologic features, and typical surgical therapies for the various types of MR.
In type I, the dominant pathologic feature causing MR is annular dilatation resulting from left ventricular dilatation. The mitral leaflets are structurally normal and demonstrate normal mobility. This form of MR is classically seen in patients with dilated cardiomyopathy, but it accompanies any form of severe MR that causes left ventricular dilatation. Annular dilatation is typically asymmetric, predominantly occurring along the attachment of the posterior leaflet. This causes the anteroposterior diameter of the mitral orifice to be greater than the transverse diameter (normal anteroposterior/transverse diameter ratio is 3:4). These alterations in annular geometry result in failure of coaptation of the mitral leaflets and typically produce a central jet of MR. Surgical correction of annular dilatation is achieved by insertion of a flexible prosthetic ring of suitable shape and size, approximating the area of the anterior mitral leaflet with a vertical height equal to the height of the anterior leaflet. By producing a measured plication of the posterior annulus, the ring attempts to restore the normal contour and function of the valve (43,44). The flexibility of the ring is thought to be important in maintaining the normal sphincteric function and saddle shape of the mitral annulus. Despite this hypothetic advantage, rigid posterior and circumferential annuloplasty rings have also been used successfully, and there are no conclusive data proving the superiority of one method over the other.
In type II, excessive motion of the leaflets occurs such that one or both leaflets will prolapse into the left atrium during systole beyond the plane of the annulus. The dominant pathologic feature underlying this type of MR is myxomatous degeneration of the leaflets and/or chordae. Older patients may demonstrate a distinct form of degenerative mitral disease termed fibroelastic deficiency in which the leaflets are thin and translucent, as opposed to yellow and thickened. Various repair techniques specifically tailored to the site of prolapse (i.e., anterior, posterior, bileaflet) have been developed (Table 79.2) (45,46). In addition to these preparative techniques, adjunctive ring annuloplasty is almost always added to the surgical procedure (43,44,47). The best surgical results are achieved in patients with posterior leaflet prolapse, who comprise the majority of patients with mitral valve prolapse.
In type III, motion of one or both leaflets is restricted. Two major pathologic features underlie this type of MR. Restricted leaflet motion may be caused by rheumatic disease (usually affecting the posterior leaflet). Surgical repair of this condition is uncommon, and replacement is usually performed. Alternatively, restriction may occur on an ischemic basis in patients with a prior myocardial infarction (typically inferoposterior), in whom remodeling and distortion of the left ventricle and papillary muscles result in displacement of the papillary muscles away from the mitral annulus and produce tethering and restriction of the mitral leaflets (48). The optimal surgical therapy for ischemic MR is controversial (49). When utilized, repair techniques for ischemic MR generally include an undersized annuloplasty ring (50,51).
TABLE 79.1 Mitral Regurgitation: Classification and Surgical Treatment | |
|---|---|
|