Fig. 4.1
Historical picture of a handmade double-lumen coaxial catheter for institution of an inferior vena cava-inferior vena cava bypass through a single surgical cutdown
In the 90s, with the advent of thin-walled spring-wire-reinforced catheters on the market, the first reports on percutaneous cannulation for cardiac and respiratory ECMO support were published [7, 8]. Since then, the percutaneous cannulation technique became the first choice in establishing vascular access for ECMO. The benefits reported were a shorter procedure time, almost null bleeding at insertion site if a coagulopathy was not present, a reduced risk of cannula-site infection, and a very simple decannulation [9].
4.3 General Considerations for the Percutaneous Placement of ECMO Cannulas
The establishment and maintenance of adequate vascular access is essential for any type of ECMO support and can be achieved by percutaneous approach outside the operating room by trained personnel (intensivists, emergency department physicians, cath-lab cardiologists). Since a failure in cannulation would be critical for ECMO institution, a cardiothoracic surgeon or a vascular surgeon must be available on site to perform a surgical approach via a semi-Seldinger or a cutdown technique if difficulties in cannulation would arise. These problems are mainly related in the literature to the placement of the arterial cannula in VA ECMO for cardiac assist.
Patient size and ECMO configuration mainly dictate the choice of the ECMO cannulas. Different cannulas are available on the market for ECMO cannulation purpose. Recently a comprehensive review on this argument was published [10], and we will only summarize some cannula features that are important to know when choosing a cannulation site.
The manufacturer provide the specifications about the pressure drops generated at different flows to help choosing the right cannula for the specific clinical need [11]. For an adult patient of >70 kg weight in a VV configuration, venous drainage cannula ranges from 23 to 25 F, while reimmission cannula from 19 to 21 F.
Vascular access for VV ECMO can be challenging in patients with a high BMI; in most of these patients, the femoro-jugular approach must be considered as the first choice. The same would apply to pregnant woman: in late pregnancy a 15–30° left lateral tilt position has been proposed to facilitate the insertion of the femoral cannula [12].
Vascular ultrasound has become invaluable for the localization of the vessels and measurements of their diameters [13, 14]. As a generic rule the size of the cannula must be no more than two-thirds of the vessel diameter, so that blood coming from the leg can flow freely around the cannula and venous drainage from the limb is not impaired. This is of greater importance when cannulation of the femoral artery is needed.
Some recent papers [15–19] describe the decision process in choosing a peripheral percutaneous approach, the cannulation techniques available, the equipment needed, and the procedure itself.
Choosing the vessels for access in VV ECMO must take into account:
The maximum ECMO flow needed for the support of the patient
The maximum recirculation tolerable
The patient’s comfort
The anatomical difficulties or the presence of some obstructed vein
The size of the drainage cannula is the main determinant of ECMO blood flow, being flow directly related to the fourth power of cannula radius; the best position would be in the intrahepatic portion of the inferior vena cava or in the right atrium. Multiple holes are distributed along the cannulas to enhance blood drainage (multiple-stage drainage cannulas). The reimmission cannulas normally have holes only in a short portion near to their extremity. There is no problem in choosing multistage cannulas both for drainage and reinfusion if a femoro-jugular approach is applied (Fig. 4.2), but if a femoro-femoral approach is chosen, a different kind of venous cannula must be used, because the side holes will generate a very high recirculation of oxygenated blood from the reimmission cannula to the drainage one (Fig. 4.2).
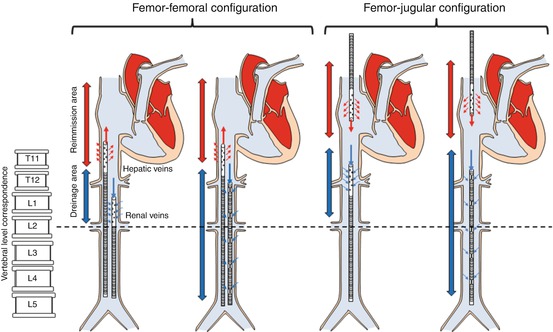

Fig. 4.2
Possible VV cannula configuration according to the type of drainage cannula (multistage side holes or cannula with side holes close to the tip) and to VV configuration (femoro-femoral or femoro-jugular). Correspondence between inferior vena cava main branches (hepatic and renal veins) and vertebral bodies is drawn. Independently from the reimmission cannula, tip of the drainage cannula should be positioned above renal veins, possibly in the intrahepatic portion of inferior vena cava. Multistage cannula should be used for drainage only especially in the femoro-femoral approach to minimize blood flow recirculation
4.4 Preparation of the Patient for Percutaneous Cannulation
Before starting the ECMO cannulation procedure, in a nonemergent situation, an arterial cannula (a right radial arterial catheter if a peripheral VA femoro-femoral ECMO must be instituted) and a central venous catheter must be inserted for monitoring. Blood specimens are drawn to assess complete blood cells count, basal coagulation profile (PT, aPTT, fibrinogen, d-dimers, and ATIII if available), blood chemistry, and blood gases. According to the results, a request for packed red blood cells, platelets, or plasma can be forwarded to the transfusion service.
The nurse in charge for the patient prepares the chosen vascular sites according to the procedure normally used for other central accesses; hair removal is performed if necessary with a hair clipper maintaining the integrity of the skin.
The cannulation procedure must be accomplished with complete aseptic technique; thus at least two operators will perform surgical hand washing and will be dressed with maximal sterile barrier precautions, cap, mask, sterile gown, and sterile gloves. The skin at insertion sites will be prepared with chlorhexidine 2 % and the surgical field prepared with large drapes covering the entire bed allowing space for cannulas and tubing during the procedures [21]. At this time a shot of antibiotic (first- or second-generation cephalosporin) is advisable as prophylaxis during the procedure [22].
The material for cannulation is prepared on a serving trolley and comprises:
A needle for venipuncture
A J-tipped guidewire
Dilatators (multiple or tapered)
Surgical tools
Sutures
4.5 VV ECMO Percutaneous Cannulation
Since the end of the 1980s, the percutaneous approach was introduced and can now be considered the first-choice technique [8, 15–19, 23, 24] for VV bypass. The surgical procedure has been almost completely abandoned, since this technique is more time-consuming and burdened with complications, uncontrollable bleeding representing the main one. The main advantage of percutaneous cannulation is a reduced risk of bleeding, but this technique also allows shorter operative time and a much easier mobilization and nursing of the patient.
Percutaneous cannulation of the femoral, jugular, and rarely subclavian vein is described in the literature. Axillary vein cannulation requires always a surgical technique.
The technique used for percutaneous cannulation is similar to the one introduced by Seldinger almost 60 years ago; for a detailed description, refer to the Lancet review published in 2005 [25].
4.5.1 Femoro-Femoral Approach
The two operators will localize the femoral veins below the inguinal ligament, and the procedure starts with the puncture of the vessels with an 18 G needle under ultrasound vision. We are used to introduce first an 8 French catheter sheath introducer into the femoral veins using the Seldinger technique and prepare a concentric purse-string suture around the insertion point in order to limit blood loss during the multiple dilatations of the vessels. A stainless-steel J-shaped guidewire (0.038 in. × 150–180 cm) is passed through the 8 Fr introducer; the guidewire must be long enough to reach the inferior vena cava. After wire placement, a 2,500–5,000 unit heparin bolus is administered to prevent thrombosis in the cannulas. At this point vessel dilators of increasing caliber are passed subsequently over the guidewire in order to obtain the right dilatation for the chosen cannula. To avoid kinking of the guidewire, it is important that the wire moves freely within the dilator, one operator will maneuver the dilators while the other will maintain the guidewire aligned with the dilator and with a slight tension. To facilitate the dilatation of the vessel and minimize the risks of guidewire kinking, our group introduced some years ago a modified technique in which three guidewires were inserted in the same vessel [26]; a dilator was passed over each wire to obtain proper dilatation for the chosen cannula (e.g., if a 24 Fr cannula has to be inserted, an 8 Fr dilator was passed over each guidewire). The development of a single progressive tapered dilator (Dilator Coons Taper 4–22 Fr, Cook Medical, Bloomington, USA) allows now to reduce the dilatation step to the passage of a single dilator if a cannula up to 21 Fr must be inserted or a two-step dilatation if a larger cannula is needed. The quality of the guidewire is also crucial for the success of the maneuver; if the guidewire is too soft, the risk of kinking while passing the dilators is very high; we have good results with the use of the Amplatz Super StiffTM Boston Scientific Guidewire.
After the proper dilatation is achieved, the cannula is inserted over its introducer; when the right position is achieved, the introducer and the guidewire are removed and a controlled filling of the cannula with blood is allowed by maintaining the extremity of the cannula slightly above the bed plane. The drainage cannula is inserted first and flushed with saline. Then the two operators move to the contralateral site, and the reimmission catheter is inserted with the same technique. Both cannulas are then secured to the skin at least in two points.
The VV femoro-femoral approach carries a higher risk of blood recirculation compared to the femoro-jugular access; for this reason it is important to put the tip of the drainage cannula at the level of L1–L2, in order to receive the blood contribution of the renal veins, while the tip of the reimmission cannula should be placed close to the junction between the inferior vena cava and the right atrium (e.g., at the level of T10–T11). In this way blood recirculation should be acceptably low, just around 10–15 % (Fig. 4.2).
A bedside imaging technique is therefore advisable to control the guidewire position and its shape during dilatations of the vessel and to guide the correct cannula position. Chest and abdominal x-rays are static and don’t allow the rapid correction of cannula position during the procedure [27]. Ultrasound and fluoroscopy can be used during cannulation to optimize catheter placement. Ultrasounds are easily accessible at the bedside; patient’s characteristics and expertise of the operator are the main determinants of adequate imaging [28]. Fluoroscopy would be the best imaging technique to visualize guidewire misplacements during cannulation [29] and therefore avoid ECMO cannula malposition, but is rarely available at the bedside and carries the risk of x-ray exposure, and with the new technological ICU beds, fluoroscopic vision of the entire procedure is sometimes very difficult.
4.5.2 Femoro-Jugular Approach
Another option to perform VV bypass is represented by the femoral-jugular approach. In this case one operator proceeds with the cannulation of the femoral vein as described above, and the other acts on the internal jugular vein. It’s advisable to drain blood from the femoral cannula positioned in the inferior vena cava in order to minimize recirculation. The return cannula is positioned through the internal jugular vein proximal to the right atrium. Cannulation of the jugular vein carries the risk of pneumothorax, and this must be taken into account in choosing this site. A shorter cannula, if available, must be chosen to allow better fixation. The increasing implementation of VV ECMO in awake spontaneously breathing patients arises the warning about the risk of air embolism during jugular vein cannulation. Therefore some groups advocate elective intubation before the procedure and extubation thereafter [30].
4.5.3 Double-Lumen (Avalon) Cannula
The “two vessel approaches,” femoro-femoral and femoro-jugular, are not comfortable for the patients. Movements are limited, and an increased need of sedatives is reported.
A single-vessel approach has been recently applied also in the adult population through a double-lumen cannula available in different sizes ranging from 13 to 31 Fr [31–34]. This type of cannula allows both drainage and reinfusion. The cannula has to be introduced through the internal jugular vein, and the placement should be guided using fluoroscopy and ultrasounds [35–37]. The position is crucial; the cannula must cross the right atrium with the tip in the inferior vena cava. Blood is drained from both the superior and inferior vena cava while the reinfusion occurs through a separate lumen into the right atrium just facing the tricuspid valve. This cannulation seems to have good results [33, 34] and allows physiotherapy with a walking patient [38].
4.6 VA ECMO Percutaneous Cannulation
Percutaneous femoral cannulation for venoarterial VA ECMO is mainly an emergent procedure and can be performed everywhere in the hospital [39] and was recently performed also outside the hospital [40, 41]. While VV cannulation for respiratory assistance generally allows time for a safe procedure, VA cannulation for cardiac rescue requires to be accomplished in the shortest possible time, exposing to potentially fatal difficulties and complications. Nevertheless, the percentage of successful cannulation is very high, hovering in many studies over 90 % [42, 43]. We already stated above that the presence of a cardiothoracic or vascular surgeon is advisable on site during the procedure; after one or two unsuccessful attempts to locate and puncture percutaneously the vessels, a switch to an open technique is mandatory.
4.6.1 Implantation Technique
The preliminary identification of the femoral vessels using ultrasound can facilitate the task and allows a more careful selection of the cannula diameter according to the size of the vessel. The procedure is best performed with two operators, to control the cannulas and wires. The placement of femoral catheters is performed under aseptic technique and begins with percutaneous puncture of the femoral vessels. If the puncture is performed during CPR, both players may act simultaneously, trying to locate the femoral artery and vein. It is preferable to use both sides for cannulation whenever possible to minimize the chance of impaired limb perfusion. If time allows it is preferable to perform an ultrasound-guided procedure. Following the Seldinger technique, a flexible J-tip guidewire (0.038 in. × 150–180 cm) is advanced from the femoral vein into the inferior vena cava (IVC) directed toward the right atrium, and an Amplatz ultra-stiff J-tip guidewire (0.038 in. × 180 cm) is advanced from the femoral artery toward the aortic valve. After wire placement, a 2,500–5,000 unit heparin bolus is administered to prevent thrombosis in the cannulas. Using a single progressive dilator (Coons Taper 4–22 F, Cook Medical, Bloomington, USA), the venous and arterial accesses are progressively dilated and cannulas are subsequently introduced over the wire. The venous cannula is advanced till the cannula tip is in the mid-right atrium. The arterial cannula is advanced for its entire length into the iliac artery. The wires are removed and the extremities of the cannulas are clamped. The lines are de-aired and connected to the ECMO circuit. Finally the cannulas are secured to the skin with sutures. The mean cannulation time is around 30 min in our series with a learning curve that determined a reduction of the time from 46 min for the first 5 patients in 2008 to 29 min for the last 15 patients in 2012. If the procedure is nonemergent, a distal perfusion catheter, or at least the guidewire, is positioned in the superficial femoral artery before the insertion of the arterial cannula. Distal perfusion of the leg is a simple and well-accepted method to increase the circulation of the cannulated leg, and several criteria to detect leg ischemia were developed [44–49]. In an elective VA ECMO procedure, the distal perfusion catheter is inserted before arterial ECMO cannula placement, since residual pulsation of the distal artery allows its easier location. In an emergent situation, like during CPR, there is no time to insert the distal perfusion catheter electively and the maneuver is deferred starting a strict control of the limb perfusion. In our experience a distal perfusion catheter to prevent leg ischemia is placed when a Doppler examination of the arteries of the leg does not detect the presence of an adequate flow downstream of the arterial cannula. We choose a 6–8 F 11 cm introducer, (Avanti+, Cordis, LJ Roden, Netherlands) as the distal perfusion catheter. This can be placed percutaneously under US guidance and connected via suitable connectors to the arterial limb of the ECMO circuit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


