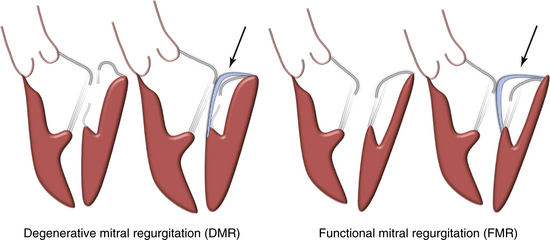
Chapter 10 Percutaneous treatment of mitral regurgitation (MR) remains one of the most important areas of innovation and unmet clinical need in the field of adult structural heart disease. It is also one of the most challenging areas for device development because of the complexity of mitral valve (MV) function and the numerous pathologies that have traditionally required highly individualized surgical corrective therapy. Mitral leaflet mobility and coaptation rely on integrated function of the mitral annulus, chordae tendineae, papillary muscles, left ventricle, and left atrium. Together these structures are referred to as the mitral apparatus (Figure 10–1). MR is an important and often underrecognized entity in which blood regurgitates into the left atrium from the left ventricle during systole. Published guidelines have established indications for surgical MV correction, generally for patients with symptoms, evidence of left ventricular (LV) enlargement or systolic dysfunction, or atrial fibrillation.1,2 These guidelines have resulted in approximately 40,000 MV operations annually in the United States and a comparable number in Europe.3 However, an unmet clinical need exists for a large population of patients with congestive heart failure–associated MR, with comorbidities, or at high surgical risk.4,5 Because MR reduction in these patients can improve quality of life and result in favorable LV remodeling, numerous percutaneous approaches for treating MR have been developed in the last decade. This chapter describes existing and emerging therapies that are rapidly assuming importance as therapeutic options for patients with MR. Figure 10–1 The mitral valve apparatus. MR is a heterogeneous disease reflecting multiple underlying etiologies that converge on systolic regurgitation of the MV. Carpentier, Barlow, and others have described three general categories of MR with associated subclassifications (Figure 10–2).6–8 Degenerative MR (DMR; Carpentier Type II dysfunction) results from structurally abnormal leaflets or chordae and occurs in fibroelastic deficiency with leaflet prolapse, myxomatous leaflet degeneration, or Barlow disease with diffuse excess tissue and chordal elongation. Over the course of 10 years, 90% of patients with severe MR caused by a flail posterior leaflet will either die or require MV surgery.9 Surgical MV repair is the preferred treatment for degenerative MR because it is associated with improved postoperative outcomes and survival when compared to MV replacement.10 Multiple surgical repair techniques exist, including leaflet repair with resection, chordal transfer, use of polytetrafluoroethylene neochordae, and prosthetic ring or band annuloplasty.11 Novel therapies addressed later in this chapter include percutaneous approaches to these leaflet and chordal repair strategies. Figure 10–2 The Carpentier classification of mitral valve regurgitation. Functional MR (FMR; Carpentier Type I or IIIb) results from underlying LV dysfunction and annular dilation, leading to impaired coaptation of otherwise structurally normal leaflets. Surgical correction of FMR improves functional class and LV remodeling,12 but it is uncertain whether this is associated with reduced mortality.13 Even with modern surgical annuloplasty techniques, up to one third of patients with FMR develop recurrent moderate or greater MR within 1 year of surgery.14–16 A number of predictors for recurrent MR after restrictive annuloplasty have been identified, including LV end-diastolic diameter greater than 65 mm,17 posterior leaflet angle of 45 degrees or greater,18 and MV coaptation depth of 11 mm or more.19 As many as 500,000 people in North America have clinically significant congestive heart failure–associated MR, but the majority of those patients are not offered any MV intervention. Given that even asymptomatic patients with severe MR have higher 5-year rates of death, heart failure, and atrial fibrillation, a significant need exists for improved FMR therapies. Many of the devices discussed in this chapter attempt to address percutaneous approaches for treatment of FMR, primarily through percutaneous annuloplasty. From an anatomic and interventional perspective, technologies for addressing MR can be categorized into treatments focused on leaflet repair, annuloplasty, chordal implants, LV remodeling techniques, and percutaneous MV replacement (Table 10–1).20 The following discussion examines interventional techniques in development for each of these approaches. TABLE 10–1 Past and Present Percutaneous Therapies for Mitral Valve Regurgitation MitraClip (Abbott Vascular Structural Heart, Menlo Park, Calif.) therapy has become an important therapeutic option for patients with MR, and important investigations centered on this therapy have given credence and momentum to the entire field of percutaneous MV repair.21 MitraClip therapy is based on the surgical edge-to-edge repair first described by Alfieri.22 Because of the wealth of data and clinical experience related to MitraClip therapy, this technique is the subject of a separate chapter in this volume (Chapter 11). The Cardiosolutions Percu-Pro system (Cardiosolutions Inc., Stoughton, Mass.) is a percutaneously delivered polyurethane-silicone polymer “Mitra-Spacer.” The spacer is balloon-shaped, occupies the regurgitant mitral orifice, and is anchored to the LV apex (Figure 10–3). The spacer has the ability to “self-center” within the regurgitant orifice. Animal studies have shown that the device is not associated with any significant inflammatory response, and early clinical trials are underway. Figure 10–3 The Percu-Pro system. Middle Peak Medical (Palo Alto, Calif.) is developing a unique solution that involves minimally invasive or transcatheter implantation of a posterior “neoleaflet.” The potential advantage of this approach is that the neoleaflet can restore coaptation with the anterior leaflet despite a variety of underlying degenerative or functional etiologies. Because the device consists only of this thin neoleaflet, the delivery profile is much smaller than a complete transcatheter mitral replacement. Since native posterior leaflet function is rendered inactive by the neoleaflet, no complex repair strategies are required. The implant does not interfere with the mitral support apparatus, so that ventricular–annular mechanical coupling is preserved. At publication, the device is undergoing preclinical development; a schematic of the technology is shown in Figure 10–4. Figure 10–4 Middle Peak medical neoleaflet. The MOBIUS device (Edwards Lifesciences, Irvine, Calif.) utilized an innovative transseptal catheter that possessed suction capability in order to grasp the leaflets. Once the leaflets were grasped, a suture was delivered to the leaflets to create a double-orifice valve. In the initial in-human experience, the procedure was performed successfully on 15 patients. Nine of the patients had acute reduction of MR by 1 grade or more, and 6 patients had sustained improvement at 30 days. This device is no longer in further development because of the lack of apparent durability in the initial studies.23 The NeoChord device (NeoChord, Inc., Eden Prairie, Minn.) utilizes a transapical approach for implantation of artificial chordae tendineae in patients with leaflet prolapse or flail (Figure 10–5). The LV apex is exposed via a left lateral minithoracotomy, and a sheath is advanced via the apex towards the MV leaflets. With the guidance of transesophageal echocardiography (TEE), a catheter is used to grasp the leaflets and attach artificial chordae, which are then anchored to the LV apex. The system utilizes a unique fiber optic feedback system to ensure that the target leaflet is appropriately centered in the jaws of the device. Once the NeoChord is attached, the operator can optimize real-time reduction in MR by varying chord tension and the extent of MV prolapse. The European Transapical Artificial Chordae Tendineae (TACT) trial is still underway at publication of this chapter and will target subjects with severe DMR. Figure 10–5 NeoChord device (NeoChord, Inc., Eden Prairie, Minn.). Most percutaneous approaches for annuloplasty have attempted to reshape the annulus “indirectly” by reshaping the coronary sinus (CS). The CS travels in close proximity to the mitral annulus (Figure 10–6). Importantly, however, the CS does not directly lie in the annulus, but rather travels 5 to 10 mm superior and diagonally to the plane of the mitral annulus.24 Any conformational change in the CS must be transmitted through sinoannular tissue, which attenuates any applied force. Additionally, the sinoannular tissue may remodel and redilate over time, thereby compromising durability of MR reduction. Several devices have been developed for placement within the CS. Each of these systems creates focal tension to reduce the circumference of the mitral annulus and the septal–lateral (SL) dimensions, with resulting reduction in MR. Although these devices are relatively easy to deploy, reproducible efficacy is a limitation because of the variable relationship of the CS to the annulus, and these devices all harbor the risk of circumflex coronary artery compression.25 Figure 10–6 Indirect mitral annuloplasty using the coronary sinus.
Percutaneous Approaches for Treating Mitral Regurgitation
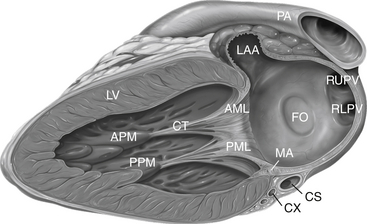
Anatomy as seen from a section though the left ventricle and left atrium. AML, Anterior mitral leaflet; APM, anterior papillary muscle; CS, coronary sinus; CT, chordae tendineae; CX, circumflex coronary artery; FO, fossa ovalis; LAA, left atrial appendage; LV, left ventricle; MA, mitral annulus; PA, pulmonary artery; PML, posterior mitral leaflet; PPM, posterior papillary muscle; RLPV, right lower pulmonary vein; RUPV, right upper pulmonary vein.
10.1 Mitral Regurgitation: Etiology, Prevalence, and Clinical Background
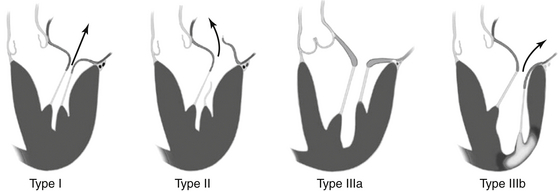
More than one type can coexist in a given individual. Type I: Normal leaflet motion with annular dilation and centrally directed jet of mitral regurgitation (MR). Type II: Increased leaflet motion (leaflet prolapse or flail) with eccentric jet of MR. Type IIIa: Restricted leaflet motion in systole and diastole, usually associated with leaflet and subvalvular thickening/scarring (rheumatic). Type IIIb: Restricted leaflet motion in systole, as seen in ischemic cardiomyopathy with history of infarct (light region in left ventricle). One or more leaflets may be “tethered” below the coaptation plane, resulting in MR.
Target of Therapy
Device Name (Manufacturer)
Mechanism of Action
Leaflet repair
MitraClip (Abbott)
Clip-based edge-to-edge repair
Percu-Pro (Cardiosolutions)
Space-occupying in regurgitant orifice
MOBIUS (Edwards)
Suture-based edge-to-edge repair
Chordal implant
NeoChord (NeoChord)
Synthetic chordae tendineae
Neoleaflet implant
Middle Peak Medical
Synthetic neoleaflet
Indirect annuloplasty
CARILLON (Cardiac Dimensions)
Coronary sinus reshaping
MONARC (Edwards)
Coronary sinus reshaping
PTMA device (Viacor)
Coronary sinus reshaping
Mitral cerclage (NIH)
Coronary sinus-right atrial encircling
PS3 System (MVRx)
Transatrial coronary sinoatrial septal shortening
Direct annuloplasty
Mitralign (Mitralign)
2 × 2 Plicating anchors through posterior annulus
Accucinch (Guided Delivery Systems)
Plicating anchors on ventricular side of mitral annulus
Cardioband (Valtech)
Plicating anchors on atrial side of mitral annulus
QuantumCor (QuantumCor)
Radiofrequency energy shrinking annular collagen
Millipede (Millipede)
Semirigid circumferential annular ring
Left ventricular reshaping
iCoapsys (Edwards)
Transventricular reshaping
BACE (Mardil)
External basal myocardial reshaping
Mitral annular anchor
M-Valve
Percutaneous anchor to allow fixation of transcatheter valve
Mitral valve replacement
Endovalve-Herrmann (Endovalve)
Valve replacement
CardiAQ (CardiAQ)
Valve replacement
Tiara (Neovasc)
Valve replacement
10.2 Leaflet Repair
Mitraclip Therapy
Other Leaflet Repair Technologies
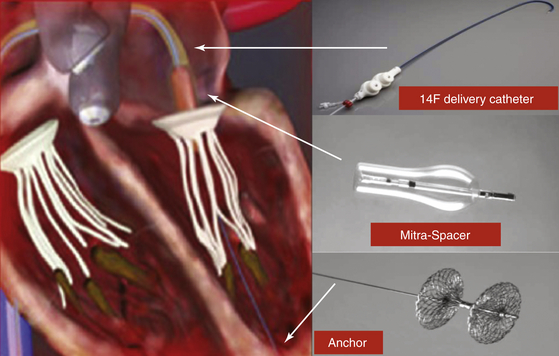
The space occupying Mitra-Spacer balloon acts as a “buoy,” filling the mitral regurgitant orifice, and is anchored by a tether to the left ventricular apex. Arrows correspond to the positions of the delivery catheter, Mitra-Spacer, and anchor.
The concept is shown by the arrows. A posterior neoleaflet (blue) is implanted and restores leaflet coaptation in either DMR or FMR.
10.3 Chordal Implantation
Neochord

The NeoChord device is shown in the left panel and consists of a catheter to grasp the leaflet, an exchangeable cartridge containing expanded polytetrafluoroethylene (ePTFE) suture, and an integrated fiber optic display to verify leaflet capture. The device is delivered from the transapical approach as shown in the right panel.
Annular Shape-Change Approaches
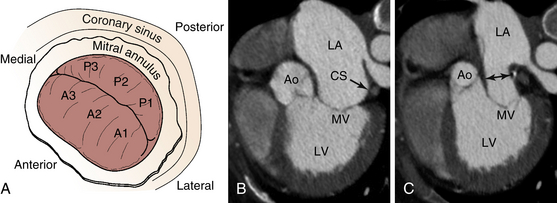
A, The CS encircles the posterior mitral annulus. B, Computed tomography scan of subject with FMR showing relationship of CS (arrow) to the MV. Note that they are not coplanar. C, Movement of the CS with device results in narrowing of the left atrial lumen above the MV (double-headed arrow), with secondary indirect decrease in the mitral annulus diameter. Ao, Aorta; CS, coronary sinus; LA, left atrium; LV, left ventricle; MV, mitral valve.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Percutaneous Approaches for Treating Mitral Regurgitation
