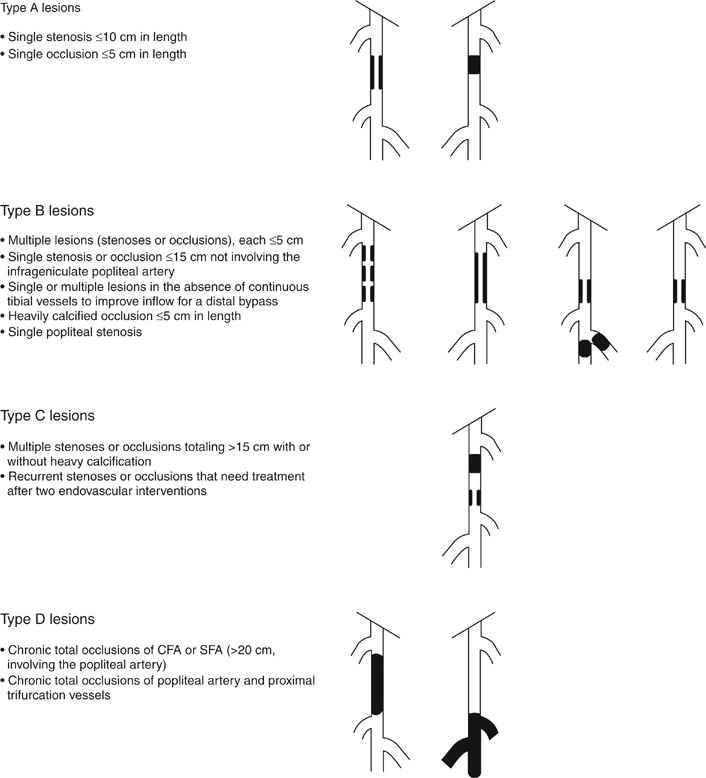
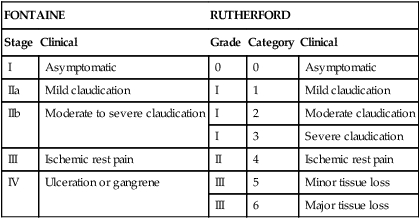
Endovascular treatment options have evolved since the 1990s to become the first-line treatment of lower extremity occlusive disease in many instances. Although initial studies showed suboptimal results, the majority of these failures were found to be related to long-segment disease, chronic occlusions, and compromised runoff. As new techniques and endovascular equipment have been added to the interventionalist’s armamentarium, outcomes have improved and enabled the treatment of ever more complex lesions. These findings are reflected in the most recent Trans-Atlantic Inter-Society Consensus Classification of femoropopliteal disease (Figure 1). Each area of infrainguinal disease should be considered separately, evaluating the experience with angioplasty with or without stenting for the superficial femoral, popliteal, and infrapopliteal arteries. A total of 219 limbs in 205 patients were included in the analysis. Patients with stenoses (78.5%) and occlusions (11%) were treated, and 6% of patients had concurrent stenosis and occlusion. Mean lesion length was 3.8 cm for stenotic lesions and 4.7 cm for occlusions. Clinical severity, classified by the Rutherford criteria (Table 1), showed a distribution of 2%, 20%, 36%, 12%, 25%, and 0% for classes I through VI, respectively. Technical success rate was 95%. Primary patency rates at 12, 24, and 36 months were 87%, 80%, and 69%, respectively. Factors found to be associated with decreased patency rates were diabetes and poor runoff score (Figure 2). The type of lesion (stenosis vs. occlusion) or the complexity of the lesion (classified according to the AHA task force classification categories 1–4) appeared to have no effect on the patency, although there were a very limited number of class 4 lesions. These patency results are higher than previously published rates. The authors attributed this to advances in equipment and techniques. They concluded that high long-term primary patency can be achieved with femoropopliteal angioplasty and that extending the spectrum of complex lesions treated by endovascular means may be warranted. TABLE 1 Clinical Classification of Peripheral Arterial Disease From Nogren L, Hiatt WR, Dormandy JA, et al: Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II), J Vasc Surg 45:S5–S67, 2007. In the RESILIENT∗ trial, 234 limbs were treated in 206 patients over a year and a half in 24 centers throughout the United States and Europe. Those included in the study had lesions shorter than 150 cm in cumulative length (if there was more than one lesion), had Rutherford class 1 to 3 clinically, and had at least one patent runoff vessel. Patients were randomized to primary stenting (134) or PTA alone (72). Mean lesion length in the stent group was 70.5 ± 44 mm and 64.4 ± 40.7 mm in the PTA group. Future directions currently being studied for treatment of femoropopliteal artery occlusive disease are the use of drug-eluting stents and covered stents. To date, a number of trials have been completed assessing the performance of drug-eluting stents with non-medicated nitinol stents. Nearly all of these trials have failed to show dramatic benefit of medicated stents in the femoropopliteal segment. Medicated stents have been evaluated in the SIROCCO∗ I and II trials as well as the STRIDES† trial. These studies have failed to prove significant benefit with the use of medicated stents over standard self-expanding nitinol stents. Using a different agent from those in either of the previous trials, paclitaxel, the most widely evaluated medicated stent is the Zilver stent (Cook, Inc., Bloomington, IN) but published results regarding this device are limited. It is likely that medicated stents add some benefit in treatment of these lesions, but it is also clear that recurrent stenosis will occur with this therapy as well. Medicated balloons have also shown some promise in reducing restenosis rates, but the application method for these devices and the types of lesions these will work best for remains to be determined. Covered stents have been used for occlusive disease in the SFA and the most widely evaluated is the Viabahn endograft (W. L. Gore & Associates, Flagstaff, AZ). In a randomized comparison of covered stenting to angioplasty, the authors noted a marked improvement in initial technical success with the use of covered stents (95% vs. 66%) and sustained improvement in patency as assessed by duplex to 1 year (65% vs. 40%). Importantly, the longer the lesion, the more dramatic the improvement in outcome seemed to be. Covered stenting likely adds significant benefit when treating long-length lesions, but longer-term patency benefit remains to be assessed, and comparison with uncovered stents is being assessed in a separate randomized trial, the VIBRANT∗ trial. Results from this trial are eagerly awaited to determine the benefit this technology has to add.
Percutaneous Angioplasty With and Without Stenting for Lower Extremity Occlusive Disease
Superficial Femoral Artery
Angioplasty
FONTAINE
RUTHERFORD
Stage
Clinical
Grade
Category
Clinical
I
Asymptomatic
0
0
Asymptomatic
IIa
Mild claudication
I
1
Mild claudication
IIb
Moderate to severe claudication
I
2
Moderate claudication
I
3
Severe claudication
III
Ischemic rest pain
II
4
Ischemic rest pain
IV
Ulceration or gangrene
III
5
Minor tissue loss
III
6
Major tissue loss
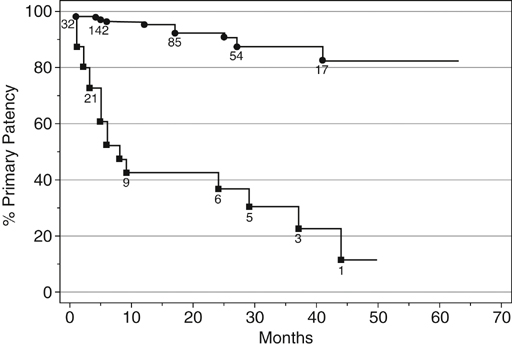
 ) and 5 or 6 (
) and 5 or 6 ( ). Numbers shown are the patients at risk at a given time. Standard Error is <10% to 50 months. Primary patency at 36 months is 87% among limbs with a score of 0–4, compared to 30% among limbs with a score of 5 or 6 (single stenotic runoff vessel or occlusion of all three tibial arteries) (p < .0001). (From Clark TW, Groffsky JL, Soulen MC: Predictors of long-term patency after femoropopliteal angioplasty: Results from the STAR registry, J Vasc Interv Radiol 12:23–33, 2001.)
). Numbers shown are the patients at risk at a given time. Standard Error is <10% to 50 months. Primary patency at 36 months is 87% among limbs with a score of 0–4, compared to 30% among limbs with a score of 5 or 6 (single stenotic runoff vessel or occlusion of all three tibial arteries) (p < .0001). (From Clark TW, Groffsky JL, Soulen MC: Predictors of long-term patency after femoropopliteal angioplasty: Results from the STAR registry, J Vasc Interv Radiol 12:23–33, 2001.)
Angioplasty with Stenting
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
