Pediatric Heart Transplantation
Robert E. Shaddy
Francesco Parisi
Pediatric heart transplantation has been practiced for over 30 years. With the advent of calcineurin inhibitors such as cyclosporine, heart transplant success rates for pediatric and adult patients have improved to the point that the initially restricted ages and indications have expanded considerably. Infant heart transplantation has been performed for over 25 years (1), and infants, children, and adolescents with complex cardiac anatomic lesions are now routinely successfully transplanted (2,3,4). There have been many additional immunosuppressive agents discovered since cyclosporine, and novel new agents are in investigational stages. The increasing experience and newer drugs promise even better long-term results. Currently, the half-life (50% still alive) for children undergoing heart transplant is approximately 11 to 18 years, depending upon age at transplantation (4). Decades-long survival is now a reality in some recipients (5).
Over 300 cardiac transplants are performed annually in pediatric patients in the United States. Many more infants, children and adolescents could benefit from transplantation each year. The rate-limiting step to making heart transplantation more widely available remains donor availability. Matching of appropriate donors to recipients is a more complicated problem in pediatrics with fewer recipients awaiting transplant at any given time compared to adults. Thus, the logistics of matching the size, blood type, and location of donor and recipient are logistically more complex.
Organ transplantation in the United States is sanctioned by congressional mandate through the National Organ Transplant Act (NOTA) of 1984. NOTA created the framework for the Organ Procurement and Transplant Network (OPTN). The contractor for the OPTN is the United Network for Organ Sharing (UNOS). The process of organ donor identification is required for all hospitals, and the management of organ donors is by government-regulated local agencies called OPOs (Organ Procurement Organizations). The decision to donate organs remains a voluntary process involving donor and family wishes. The current UNOS allocation algorithm has three status categories based on medical urgency. These categories are status IA, IB, and II. Status IA is for the sickest patients needing an urgent transplantation for survival. Waiting mortality is high for status I patients and remains a significant problem in all age groups (6,7,8). Even within the sickest pediatric wait-list group (status 1A), there is large variability in outcomes, with those requiring extracorporeal membrane oxygenation (ECMO) having the highest wait-list mortality (7,9). The synchronization of recipient need, donor availability, consent for organ donation, and finally organ transplantation is a modern medical miracle that represents the ultimate in human sharing.
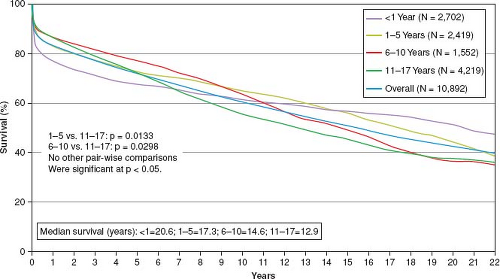
At present, survival rates at 1 year in excess of 85% and at 5 years of more than 70% can be expected following pediatric heart transplantation (Fig. 64.1). Catch-up growth and hemodynamic rehabilitation to normal childhood functional status is the norm. The quality of life can be and often is normal. Heart transplantation remains the only hope for children with lethal cardiomyopathy,
some forms of complex congenital heart disease, and some infants and children with failed surgical interventions. This chapter discusses the indications for heart transplantation, various phases of the transplant process (preoperative, early postoperative, and late), the immunosuppressive drugs, the role of heart and lung transplantation, and the issue of retransplantation.
some forms of complex congenital heart disease, and some infants and children with failed surgical interventions. This chapter discusses the indications for heart transplantation, various phases of the transplant process (preoperative, early postoperative, and late), the immunosuppressive drugs, the role of heart and lung transplantation, and the issue of retransplantation.
Pretransplant Evaluation
A large amount of historical, anatomic, hemodynamic, metabolic, immunologic, and psychosocial information is required before deciding whether cardiac transplantation is suitable for a given child (10) (Table 64.1). A comprehensive history and physical examination is mandatory, including age, height, weight, and body surface area. Since pediatric heart donors are matched with recipient size, accurate measurements of the recipient are critical and need to be continually updated in those who wait long periods of time and undergo changes in their height or weight. Cardiac diagnoses, including all previous surgeries, must be meticulously delineated, with particular attention to venous and arterial connections, since the surgeon will need this information in order to devise a surgical plan in those with complex congenital heart disease with abnormal connections. The use of extended donor heart and vessel retrieval and creative intraoperative techniques has resulted in successful orthotopic heart transplantation in children with abnormal situs and/or significant systemic and pulmonary venous anomalies (2,11). Immunization status should be determined, and if incomplete prior to listing for transplant, immunizations may be given as indicated by age (12,13). A history of malignancy, once considered to be an absolute contraindication to transplantation, may not preclude transplantation in selected patients (14,15). A thorough laboratory evaluation is necessary to determine liver and kidney function since severe, irreversible liver or kidney dysfunction would generally exclude the child from consideration for heart transplantation, although some centers may consider multiple organ transplants. An equally extensive infectious disease evaluation is necessary to exclude active infection, to determine potential latent infections such as cytomegalovirus (CMV) or Epstein–Barr virus (EBV), and to provide baseline data on susceptibilities that can be followed serially after transplant. An accurate and documented blood type is critical since this is usually the main compatibility factor used for donor/recipient matching.
TABLE 64.1 Routine Pretransplant Evaluation | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Evaluation of the immune system is an important part of the pretransplant evaluation. Although it is now the standard to check recipient and donor human leukocyte antigen (HLA) status, this information is not usually part of the decision-making process when determining donor suitability. Retrospective studies have suggested that HLA compatibility is rare but may lessen rejection and improve graft survival in heart transplantation (16). In part due to the constraints of cold ischemic time (optimally less than about 4 hours) and the ongoing organ donor shortage, prospective HLA matching is not routinely performed in heart transplantation. Panel reactive antibodies (PRAs), a laboratory measure of preformed anti-HLA antibodies, are assayed to determine whether the recipient has any circulating anti-HLA antibodies before transplant. Recipients with circulating donor-specific anti-HLA antibodies before transplant have decreased graft survival when compared to those without these antibodies (17,18). Patients with a significantly elevated PRA before transplantation have traditionally undergone a prospective crossmatch between donor and recipient before acceptance of a donor organ (19,20,21). However, this can severely limit the donor pool available to a recipient and increases mortality waiting for transplant in those awaiting a compatible donor (17,21). Because of the significant difficulties associated with finding a compatible crossmatch in patients with elevated PRAs, multiple treatment modalities, including intravenous immunoglobulin (IVIg), plasmapheresis, cyclophosphamide, rituximab, and most recently, the proteasome inhibitor bortezomib have been utilized with variable degrees of success to try to decrease the pretransplant PRA of highly sensitized patients (22,23,24,25,26,27,28,29).
With the exception of some infants with unoperated congenital heart disease (e.g., hypoplastic left heart syndrome [HLHS]), most children will require a cardiac catheterization before heart transplantation. Cardiac catheterization and angiography should be performed as part of the pretransplant evaluation by someone experienced in the diagnosis and treatment of pediatric cardiovascular disease and heart transplantation. Especially in patients with complex congenital heart disease, hemodynamic and anatomic assessments are critical for appropriate pretransplant evaluation. In addition to precise anatomic and hemodynamic definition, it is necessary to determine whether other pharmacologic, catheter interventional, or surgical options may be necessary prior to transplantation. Patients with univentricular physiology, particularly those who have undergone multiple palliative procedures, are a
unique group of patients whose pretransplant evaluation can be very complicated. For example, children after the Fontan operation may have many complications such as dysrhythmias, protein-losing enteropathy, cirrhosis, and/or low cardiac output that may bring them to transplant consideration.
unique group of patients whose pretransplant evaluation can be very complicated. For example, children after the Fontan operation may have many complications such as dysrhythmias, protein-losing enteropathy, cirrhosis, and/or low cardiac output that may bring them to transplant consideration.
Assessment of pulmonary arterial anatomy, pressures and, when possible, pulmonary vascular resistance is critically important in the pretransplant evaluation of most children being assessed for heart transplantation. Severe, fixed elevation of the pulmonary vascular resistance is a contraindication to orthotopic heart transplantation because of concerns of acute posttransplant right ventricular failure. Both elevated transpulmonary pressure gradient and elevated pulmonary vascular resistance have been identified as risk factors for early mortality after heart transplantation (30). However, a previous multi-institutional analysis of risk factors for mortality in children >1 year of age at the time of transplantation did not find elevated pulmonary vascular resistance to be a risk factor (31). The current selection criteria for pediatric orthotopic heart transplant recipients exclude those patients with significantly elevated nonreactive pulmonary vascular resistance (3,10). In these patients who are denied orthotopic heart transplantation, other options such as heterotopic heart transplantation, heart/lung transplantation, or lung transplantation with repair of the congenital heart defect may be considered (32,33,34). More recently, some centers have provided data supporting the possibility for accepting children for heart transplant alone when PVR is much higher than 6 Wood units (35). Accurate evaluation of the degree of pulmonary hypertension may not be possible in those patients with either discontinuous pulmonary arteries or multiple sources of pulmonary blood flow, or in those with multiple branch pulmonary artery stenoses. Several agents have been shown to have both acute and chronic beneficial effects in lowering transpulmonary gradients and pulmonary artery pressures in adults and children. Response to these agents, including intravenous nitroglycerin, nitroprusside, prostaglandin E1, dobutamine, enoximone, milrinone, in addition to inhaled nitric oxide, has been shown to predict outcome after heart transplantation (36,37,38,39,40,41). Mechanical circulatory support can also be considered in refractory cases (42,43). Children with restrictive cardiomyopathy appear to be at higher risk for development and rapid progression of significant pulmonary hypertension and thus require careful monitoring and possibly early consideration for heart transplantation (44,45,46) (see Chapter 56).
Assessment of cardiac anatomy and function by a complete Doppler echocardiogram is a necessary part of the pretransplant evaluation. Cardiac magnetic resonance imaging may also be beneficial in selected patients. Endomyocardial biopsy may be indicated in certain instances, for example, to exclude active myocarditis or myocardial infiltrative diseases. Electrocardiograms and 24-hour continuous ambulatory electrocardiograms may be important in determining underlying rhythm, evidence of ischemia or previous infarction, and abnormal rhythms or intervals. A chest radiograph may be very useful for measuring the degree of recipient cardiomegaly to help in determining size limitations in potential donors. In older children, pulmonary function tests may be important, especially if there is any concern of chronic lung disease. In those who can cooperate, measurement of maximal O2 consumption may be very useful for quantifying the degree of cardiorespiratory compromise the patient is experiencing. A significantly reduced maximal O2 consumption <50% of that predicted for age may be considered evidence of compromise that should at least lead to consideration of heart transplantation as a therapeutic option (10,47,48). This diagnostic test may be less useful in those children with heart failure who have undergone the Fontan operation, since a significant number of patients in this group is unable to achieve maximal aerobic exercise capacity (49).
Psychosocial evaluation is a critical part of the pretransplant evaluation. A stable family support system that is emotionally and intellectually able to provide medications and posttransplant care is crucial to the success of the heart transplant. In many instances, it is necessary for the family to relocate to be in close proximity to the transplant center for the entire waiting period before transplantation and for 3 to 6 months after the transplant. This often provides additional stressors to the family. It is uncommon to have an absolute psychosocial contraindication to pediatric heart transplantation. However, a family history of noncompliance, substance abuse, or child abuse or neglect may be a relative contraindication to transplantation. In some instances in which the patient’s parents have been determined to be incapable of handling the responsibility of caring for the child before and after transplantation, it has been necessary for a relative to take over those responsibilities (12). Financial needs and resources can vary considerably and should be thoroughly evaluated.
Pretransplant Management
Once a patient is under consideration for transplantation, every effort must be made to stabilize or improve the patient’s clinical status. Since the waiting time for donors is unpredictable, patients may wait for long periods of time, during which time ongoing pharmacologic, catheter interventional, and occasional surgical treatments must be used as needed. Patients may deteriorate rapidly while waiting for a suitable donor, in which case, more invasive measures may be necessary to bridge the patient to transplantation. Optimization of pretransplant nutritional status constitutes a strategy to reduce waitlist mortality in this age range (50). Early intervention may be the key in improving nutritional status and outcomes for patients both before and after transplantation (51).
The epidemiology of infant heart transplantation has changed through the years as the results for staged repair of complex congenital heart disease have improved and donor resources have remained stagnant. Heart transplantation, because of donor limitations, has generally been consigned as primary therapy to those few infants with lesions such as HLHS deemed unsuitable for staged reconstruction (see Chapter 46). Primary transplantation has remained available in some centers as a parental choice, and as the only solution for the occasional young infant with profound cardiomyopathic disease and inoperable complex congenital heart disease, including some tumors. Infants awaiting heart transplantation with ductal-dependent lesions (e.g., HLHS variants or other ductal-dependent lesions without good surgical options), are critically ill, chronically instrumented, and usually in an intensive care setting while they await transplantation (52,53). Since waiting times for donors has increased at most institutions, there are increased challenges and problems associated with keeping these infants stable, sometimes for several months, before a suitable donor becomes available (54,55). Initial efforts must be directed toward opening and maintaining patency of the ductus arteriosus through the use of continuous infusion of prostaglandin E1. Once unrestricted ductal patency is achieved, therapy must be directed toward maintaining adequate systemic blood flow, sometimes through pharmacologic manipulation of the pulmonary vasculature (56,57). Some infants with HLHS and other ductal-dependent lesions have undergone percutaneous or surgical stenting of the ductus arteriosus in order to maintain ductal patency (58,59). The development of the so-called hybrid procedures has allowed surgical bilateral branch pulmonary artery banding and transcatheter stenting of the ductus arteriosus in place of a stage one procedure (60). If necessary, heart transplantation after the hybrid procedure can be performed with acceptable results (61). Up to 50% of infants with HLHS may develop a critical restriction at the atrial septal defect level over time. These infants have excessive cyanosis and hemodynamic instability and represent a high-risk group of infants who can be stabilized with interventional catheter procedures (62).
Heart transplantation has become a possible alternative to a high-risk Fontan operation in a strategy of staged palliation for
single ventricle physiology. Heart transplantation should be considered as an alternative to Fontan completion in the decision-making algorithm for high-risk Fontan candidates, since rescue heart transplantation after early Fontan failure is associated with poor outcomes (63,64,65,66,67,68). Many patients with Fontan failure evaluated for heart transplantation have abnormal liver findings by imaging modalities such as computed tomography (CT) scan, with cirrhosis in half of the patients. However, liver cirrhosis identified by CT imaging may not be an absolute contraindication to heart transplantation alone in this population (69,70,71).
single ventricle physiology. Heart transplantation should be considered as an alternative to Fontan completion in the decision-making algorithm for high-risk Fontan candidates, since rescue heart transplantation after early Fontan failure is associated with poor outcomes (63,64,65,66,67,68). Many patients with Fontan failure evaluated for heart transplantation have abnormal liver findings by imaging modalities such as computed tomography (CT) scan, with cirrhosis in half of the patients. However, liver cirrhosis identified by CT imaging may not be an absolute contraindication to heart transplantation alone in this population (69,70,71).
Patients with end-stage biventricular congenital heart disease represent a complex group for heart transplantation and require careful evaluation and management to ensure optimal perioperative and long-term outcomes. The vast majority of patients with biventricular congenital heart disease has undergone prior cardiac surgical procedures. Indications for transplantation in this subgroup are primarily progressive refractory heart failure following prior cardiac surgical reconstructive procedures. Contraindications to transplantation mimic those for other forms of end-stage heart disease (10,72,73).
The improved outcomes in surgical correction and palliation in children with CHD have led to an increasing population of adults with CHD who may develop complications and indications for heart transplantation. The most common cause of morbidity and mortality in adults with congenital heart disease is late myocardial dysfunction. As a consequence, an estimated 10% to 20% of patients suffering from congenital heart disease may eventually require heart or heart–lung transplantation. These patients have unique characteristics that can make clinical management and assessment for cardiac transplantation challenging. Survival in adults with congenital heart disease after transplantation is improved if the transplant is performed at a high-volume center, particularly those that perform pediatric transplants. The availability of pediatric heart transplant teams at high-volume transplant centers should be considered when arranging for transplantation in an adult who has congenital heart disease (74).
While HLA sensitization is uncommon in patients with cardiomyopathy, it can frequently be seen in patients with congenital heart disease who have had prior surgeries. It is well known that the use of cryopreserved allograft material induces an immune response with the development of both class I and II anti-HLA antibodies and elevated PRA (75,76). Allosensitization or highly sensitized patients are usually defined as having an elevated PRA >10%. In addition to allograft exposure, blood transfusions, mechanical circulatory support, pregnancy, and prior heart transplant have also been shown to be risk factors for developing anti-HLA antibodies (24). Transplantation in the setting of allosensitization carries increased risk and mortality. Given this increased risk, some centers may choose not to offer heart transplant to patients with elevated PRA. Alternatively, desensitization (decreasing the circulating anti-HLA antibodies) or prospective/virtual crossmatching may be alternatives to improve outcomes. Many studies have reported methods to desensitize patients, including administration of IVIg, plasmapheresis, and use of cyclophosphamide or mycophenolate mofetil (22,23,24). In addition, newer medications, including rituximab (a monoclonal antibody to CD20) and bortezomib (a proteasome inhibitor directed against plasma cells) have been shown to reduce circulating antibodies (25,26,27,28,29). Unfortunately, prospective crossmatching can be time consuming and requires the presence of both recipient serum and donor cells to perform a direct assessment of the donor-recipient crossmatch. This can be limited by geographical proximity. Alternatively, many advocate for the use of a virtual crossmatch in which the recipient anti-HLA antibody profile is compared to the donor HLA typing to predict a possible crossmatch, alleviating the geographic restrictions placed by the direct, prospective crossmatch (77,78).
Patients with heart failure secondary to ventricular dysfunction represent a significant proportion of children who are referred for heart transplantation. The natural history of dilated cardiomyopathy in children is quite variable; thus the optimal therapy for dilated cardiomyopathy and timing for transplantation in these children is unknown. Large, multicenter, randomized studies in adults with chronic heart failure have shown a significant improvement in left ventricular performance, symptoms, and survival in patients receiving angiotensin-converting enzyme (ACE) inhibitors and beta-adrenergic receptor (beta)-blockers such as carvedilol or metoprolol when compared with placebo controls (79,80,81,82). Addition of β-blockers to chronic heart failure therapy in some children (particularly those with a systemic left ventricle) may improve ventricular function, symptoms, and survival, thus delaying or even precluding the need for transplantation (83,84,85,86). ECMO has been used successfully in children as a bridge to transplantation, but remains problematic due to complications such as sepsis, bleeding, and neurologic injury (9,87,88,89,90,91). Options for mechanical support in children include miniaturized intra-aortic balloon pumps, ECMO, centrifugal pumps, and, more recently, both pulsatile ventricular assist devices (VADs) and axial flow devices (see Chapter 25). ECMO remains the most common form of mechanical support available and is the best option for acute decompensation. ECMO provides total cardiopulmonary support, can be relatively quickly accomplished, and allows the flexibility of peripheral and central cannulation (89,90). ECMO pumps, however, achieve nonpulsatile flow, and the circuit is complex. The incidence of bleeding and infection is high, and neurologic impairment with extended use is also common. ECMO also restricts mobility, impairing physical rehabilitation. VADs have potential advantages over ECMO as a mechanical bridge (92). In addition to improving a patient’s hemodynamic status and reversing end-organ dysfunction, they can be partially or fully implanted and allow physical rehabilitation to improve the patient’s condition and likelihood for successful transplantation. Biventricular VAD support can effectively be used in children as a bridge to heart transplantation and can be accomplished with low mortality and morbidity. Biventricular VAD support may offer an additional means to reverse extremely elevated pulmonary vascular resistance (43,93,94,95,96,97). However, the high rate of morbidity emphasizes the importance of optimizing the decision-making process and, particularly, the timing of implantation. The Berlin Heart has allowed significant increase in the number of children with end-stage heart failure who can be successfully bridged to transplant and the length of time they can be supported, but the total number of transplants has not increased (98). However, the persistent high rate of morbidity emphasizes the importance of optimizing the decision-making process and, particularly, the timing of implantation. Besides, many patients can develop new anti-HLA antibodies while on VAD support but the immediate impact of these antibodies appears to be limited (99). Patients with congenital heart disease and end-stage heart failure currently have a limited number of options for long-term mechanical circulatory support.
Donor Issues
Because of the ongoing donor shortage for pediatric heart transplantation, transplant cardiologists have made great efforts to maximize donor usage. Although optimal donors have normal cardiac anatomy and function, ideal size and blood type match, and minimal ischemic time, many successful pediatric heart donors are used that do not meet these ideal criteria (1). Brain death has been shown to have a deleterious effect on ventricular function with the right ventricle being particularly susceptible. Therefore, the goals of treatment following brain death are to preserve ventricular function and prevent further myocardial damage. Intensive care management usually focuses on optimizing intravascular volume status, maintaining cardiac output with the lowest amount of inotropic support required and increasing the suitability of the hearts for transplantation. Finally, donor hormonal therapy in the pediatric population can have a positive impact on survival post transplant. Many perceived risk factors often used to turn down a donor heart do not adversely affect overall recipient survival post transplant. These data highlight the importance of understanding the interaction between the donors’ and recipients’ factors across the pediatric age spectrum (100). Specifically, the use of donor hearts with
depressed ventricular function and/or elevated troponin levels may be considered in selected pediatric patients (101,102). It has been known for some time that a certain degree of both systolic and diastolic dysfunction in the donor heart can be tolerated (1,102,103). Studies have shown successful pediatric heart transplant outcomes after donor ischemic times as long as 8 hours, with no significant differences in outcomes between those with donor ischemic times >8 hours and those with donor ischemic times ≤90 minutes (104). Although the mechanism is unclear, the use of advanced-age donor hearts (>40 years of age) for appropriately sized teenage recipients carries a significantly higher 1-year posttransplant mortality than use of younger donor hearts (105).
depressed ventricular function and/or elevated troponin levels may be considered in selected pediatric patients (101,102). It has been known for some time that a certain degree of both systolic and diastolic dysfunction in the donor heart can be tolerated (1,102,103). Studies have shown successful pediatric heart transplant outcomes after donor ischemic times as long as 8 hours, with no significant differences in outcomes between those with donor ischemic times >8 hours and those with donor ischemic times ≤90 minutes (104). Although the mechanism is unclear, the use of advanced-age donor hearts (>40 years of age) for appropriately sized teenage recipients carries a significantly higher 1-year posttransplant mortality than use of younger donor hearts (105).
The incidence of sudden infant death syndrome (SIDS) has decreased over the last 20 years. There has been no difference in clinical outcome between infants transplanted with a donor who died from SIDS compared to donors who died of other causes (1,106). The proportion of transplanted infant donor hearts where donor cause of death was SIDS increased from just over 4% in 2000–2002 to nearly 9% in 2006–2008. This is possibly due to increasing confidence with the use of SIDS donors. There has been investigation into the use of non–heart-beating heart donors after cardiocirculatory death as an additional source of donors for both adults and children (107,108). There have also been recent reports of satisfactory outcomes from supporting recipients of otherwise unacceptable donor hearts with ECMO (109).
Abo-Incompatible Heart Transplantation
Blood group matching has traditionally been considered critical for heart transplantation. Since infants usually lack preformed blood group antibodies (isohemagglutinins), ABO-incompatible heart transplants have been successfully performed in infants <1 year old, and occasionally older patients (110,111,112,113). Cardiac transplantation in infancy using ABO-incompatible donors in the appropriate setting has now become an established protocol in many centers. Those infants who received an ABO-incompatible graft usually (but not always) fail to develop antibodies against the incompatible blood group epitope from the donor, while making antibodies normally to other incompatible blood groups (111,114). For example, a recipient whose blood type is O and receives a heart from a B donor will later make antibody to blood group A but usually not to blood group B. This observation has been used as an example of B-cell tolerance (115). This ABO-incompatible approach has resulted in improved wait-time survival of infants in some but not all studies (116,117). This difference may reflect the fact that donor hearts are still offered to ABO-compatible recipients before ABO-incompatible recipients in the UNOS system in the United States, but not in other systems. The 10-year outcomes of infants receiving ABO-incompatible heart transplants are virtually identical to ABO-compatible heart transplants (114). Thus, there appears to be no contraindication to listing all infants with low isohemagglutinin titers for ABO-incompatible heart transplant.
Postoperative Management
General Considerations
The postoperative course after heart transplantation can be complicated. Potential complications relate both to the donor and the recipient. Myocardial injury and cause of death, donor versus recipient size, donor heart ischemic time, blood and tissue compatibility, infectious status of both donor and recipient, recipient diagnosis, and recipient clinical and psychosocial conditions may all affect myocardial performance and postoperative course. The influence of donor and recipient genetics on this process is still being delineated. With an increasingly diverse set of transplant immunosuppressive agents available, a pharmacogenetic effect on clinical outcomes may have important implications for drug selection in the future (118).
The effects of brain injury and death on myocardial performance have been investigated (103,119). The process of brain death leads to myocardial dysfunction and is often due to multiple factors: brain death itself may cause myocardial dysfunction; the cause of death (sepsis, trauma, etc.) can directly depress myocardial contractility; and the high catecholamine environment of stress or the pharmacologic support of the donor can lead to receptor downregulation. Although no specific relationship with survival has been demonstrated, it is common for many centers to accept some degree of donor heart systolic or diastolic dysfunction, either or both of which are often reversible after transplantation. Donor ischemic times in adult and pediatric heart transplantation have been reported by many centers to increase the postoperative need for inotropic support but to not be a risk factor for 1-year mortality (104,120,121,122).
As described above, ABO-incompatible heart transplants have been increasingly utilized. This practice requires particular attention to postoperative management including specific immunosuppression and transfusion protocols (110,111). In the adolescent age group, the number of patients with congenital heart disease who become transplant candidates after a long surgical and blood transfusion history is increasing. These patients represent an increasingly HLA-sensitized heart transplant population who require special consideration and often require pre- and postoperative immunomodulatory treatment (17,24,123). It has been known for decades that the presence of circulating anti-HLA antibodies before transplantation is associated with increased risk of rejection, coronary artery vasculopathy (CAV), graft dysfunction, and death after transplantation (17,124,125,126,127,128). Injury to the graft may be acute with hemodynamic dysfunction or more chronic manifesting as chronic rejection or CAV. Donor-specific anti-HLA antibodies may be preformed due to allosensitization prior to transplant, or develop de novo at any time following transplant. The de novo development of anti-HLA antibodies after heart transplantation correlates with decreased long-term survival (129). Patients with de novo antibodies appearing more than 1 year following transplantation have the poorest survival (130).
Patients with congenital heart disease present additional perioperative problems related to their specific morphology, previous surgical procedures, and reconstructive surgery. Heart transplantation in children with an anatomic or physiologic single lung has been successfully performed, but pulmonary artery reconstruction increases the risk of mortality (131,132,133). Heart transplantation for structural congenital heart disease with single ventricle physiology is associated with substantial early mortality, and transplantation after the acutely failing Fontan may be prohibitively risky (64). Fontan status remains a risk factor of mortality after heart transplantation with an expected 5-year survival barely approaching 70%, with particularly increased risk in those with evidence of pulmonary vascular disease or even a failing Fontan circuit with preserved ventricular function (67,134,135). Tailoring of immunosuppressive therapy is a crucial issue in these patients since they are often immunocompromised from their failing Fontan physiology with protein loss, liver dysfunction, and low cardiac output. Transplant outcomes for patients after the Fontan operation are better in those who require heart transplantation because of ventricular dysfunction (rather than those with preserved ventricular function and a failing Fontan circuit) and those without significant comorbidities such as liver cirrhosis or chronic malnutrition (67). Protein-losing enteropathy, a severe complication of Fontan physiology can usually be improved by heart transplantation (65,67,136).
Practical considerations
Adequate monitoring of the postoperative heart transplant patient is essential. Guidelines from the International Society for Heart and Lung Transplantation (ISHLT) for the perioperative monitoring of both adult and pediatric heart transplant recipients are detailed in
Table 64.2 (137). Of these, standard pediatric monitoring would include all except assessment of pulmonary arterial wedge pressure and cardiac output via invasive catheters owing to concerns of catheter size and maintaining appropriate catheter position, especially in smaller recipients. However, continuous, direct measurement of pulmonary artery pressures is monitored in some pediatric patients, particularly those with elevated pulmonary arterial pressures before transplant. Perioperative hemodynamic instability can be a result of multiple causes including graft reperfusion injury, inflammatory response after cardiopulmonary bypass, elevated pulmonary vascular resistance, and labile fluid status. Most patients can be supported with catecholamine infusions after transplantation and often benefit from an elevated heart rate. Temporary pacing is also used. Milrinone is often used to reduce pulmonary and systemic vascular resistance and potentially provides a nonadrenergic-receptor–dependent form of inotropic support. The donor right ventricle is not “prepared” to deal with elevated pulmonary vascular resistances, thus some degree of right ventricular failure is common and usually lasts for several days. Many agents, such as prostaglandins, prostacyclin, nitroprusside, inhaled nitric oxide, and others have been proven to be effective in these patients (40,41). In rare instances, right heart failure may be so severe that ECMO support is required. Hemodynamic parameters, such as right-sided filling pressures and functional right ventricular assessment with echocardiography can be used to follow the course of right ventricular recovery and direct appropriate weaning from supportive measures. Preventive therapy with selective vasodilators as well as the availability of mechanical assist devices during and after heart transplantation can reduce deleterious effects of both transitional pulmonary hypertension and primary graft failure (122,138).
Table 64.2 (137). Of these, standard pediatric monitoring would include all except assessment of pulmonary arterial wedge pressure and cardiac output via invasive catheters owing to concerns of catheter size and maintaining appropriate catheter position, especially in smaller recipients. However, continuous, direct measurement of pulmonary artery pressures is monitored in some pediatric patients, particularly those with elevated pulmonary arterial pressures before transplant. Perioperative hemodynamic instability can be a result of multiple causes including graft reperfusion injury, inflammatory response after cardiopulmonary bypass, elevated pulmonary vascular resistance, and labile fluid status. Most patients can be supported with catecholamine infusions after transplantation and often benefit from an elevated heart rate. Temporary pacing is also used. Milrinone is often used to reduce pulmonary and systemic vascular resistance and potentially provides a nonadrenergic-receptor–dependent form of inotropic support. The donor right ventricle is not “prepared” to deal with elevated pulmonary vascular resistances, thus some degree of right ventricular failure is common and usually lasts for several days. Many agents, such as prostaglandins, prostacyclin, nitroprusside, inhaled nitric oxide, and others have been proven to be effective in these patients (40,41). In rare instances, right heart failure may be so severe that ECMO support is required. Hemodynamic parameters, such as right-sided filling pressures and functional right ventricular assessment with echocardiography can be used to follow the course of right ventricular recovery and direct appropriate weaning from supportive measures. Preventive therapy with selective vasodilators as well as the availability of mechanical assist devices during and after heart transplantation can reduce deleterious effects of both transitional pulmonary hypertension and primary graft failure (122,138).
TABLE 64.2 ISHLT Guidelines for Post–Heart Transplant Monitoring | ||||||||
|---|---|---|---|---|---|---|---|---|
|
The early posttransplant period (<30 days) is the most hazardous. Primary graft failure and early morbidity are largely explained by recipient issues that increase perioperative risk (112). Review of the ISHLT Scientific Registry details several major risk factors for 1-year mortality (4):
ECMO, age <1 year (hazard ratio: 3.24)
Retransplant (hazard ratio: 2.15)
On dialysis (hazard ratio: 2.06)
Congenital diagnosis (hazard ratio: 1.82)
ECMO age >1 year (hazard ratio: 1.66)
On ventilator (hazard ratio: 1.55)
Donor cause of death cerebrovascular accident versus head trauma (hazard ratio: 1.54)
Transplant year 2004–2005 versus 2002–2003 (hazard ratio: 1.50)
Postoperative bleeding can be significant in children following heart transplantation. The causes are multifactorial and include previous congenital heart surgery necessitating extensive dissection, cardiopulmonary bypass, multiple suture lines, pretransplant heparinization for VAD or ECMO support, hepatic malfunction, and poor preoperative nutritional status. Platelet and fresh frozen plasma infusions should be used as necessary to control hemorrhage, and recombinant factor VII may be useful for refractory bleeding. Volume resuscitation including packed red blood cells (preferably leukocyte-reduced and CMV negative) may be necessary, but should be administered with caution given the potential increased risk for allosensitization from transfused leukocytes, which may express non–donor-matched HLA antigens. Patients with refractory hemorrhage or those demonstrating clinical evidence of cardiac tamponade should be surgically explored.
Acute renal failure occurs postoperatively in 3% to 10% of transplant recipients (139). Hemodialysis may be necessary for refractory fluid overload and oliguria in the presence of a rising serum creatinine. Multidisciplinary team management including nephrology consultation is often useful in this circumstance. Patients often develop systemic hypertension in the immediate postoperative period. This can be secondary to baroreflex-mediated hypertension, catecholamine dysregulation from low cardiac output before transplant, significant pre-existing renal injury, and newly initiated immunosuppressive medications such as corticosteroids or calcineurin inhibitors. Nitroprusside, calcium channel blockers, ACE inhibitors, hydralazine or a combination of these usually provide adequate blood pressure control. Recipient/donor size mismatching can also influence postoperative course. “Big heart syndrome” results when the donor size is significantly larger than the recipient. In the early transplant period, donor/recipient weight ratio mismatches of >2 may result in systemic hypertension syndrome with associated central nervous system symptoms including seizures and coma. Treatment consists of antihypertensive medication titration to achieve a normal blood pressure for age. Conversely an inappropriately small donor heart size has been associated with increased mortality, and a donor/recipient weight ratio <1 has been reported as a significant predictor of fatal postoperative heart failure (140). Postoperative pericardial effusions develop in 9% to 21% of adult recipients (141,142). The incidence in pediatric patients is unknown but is likely similar to adults and may, in part, be related to an increased pericardial volume created after a dilated heart is replaced with normal-sized heart. Unless the effusion is hemodynamically compromising or there is a strong suspicion of an infectious etiology, the effusion usually does not require surgical or percutaneous drainage and can be monitored serially by echocardiography. Sinus node dysfunction is common with a reported prevalence as high as 44% (143) and is likely related to myocardial ischemia and surgical manipulation. The ISHLT guidelines recommend pharmacologic treatment or pacing to maintain an adequate heart rate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree