Fig. 2.1
Chest wall anatomy (Netters)
Pathophysiology
The etiology of pectus excavatum remains unknown. There is a strong familial tendency with up to 43 % of presenting cases with a positive family history [14–16]; however, the exact genetic link is yet to be elucidated, and the inheritance pattern is likely multifactorial [17–19]. Pectus excavatum can be a part of many genetic syndromes , the most frequently observed being Marfan Syndrome and Noonan Syndrome [20]; however, less than 1 % of patients with pectus excavatum have an underlying connective tissue disorder [21].
There have been many historical theories regarding the pathogenesis of pectus excavatum including abnormal in utero diaphragmatic development, abnormal embryonic positioning resulting in increased intrauterine pressure on the sternum, and the sequelae of systemic diseases such as syphilis and rickets [4]. Current hypotheses on the etiology of pectus excavatum focus on abnormal metabolism and overgrowth at the sternocostal cartilage resulting in weakness and instability at the joint [22]. Histological analysis of the sternocostal cartilage in patients with pectus excavatum reveals evidence of premature aging of the cartilage, abnormalities in the trace element content, namely decreased zinc, and decreased chondrocyte activity [23–25]. Relative to control specimen, cartilages from patients with pectus excavatum demonstrate decreased biomechanical stability, which is hypothesized to be the result of disordered arrangement and distribution of collagen [26].
Presentation
Pectus excavatum can be present at birth; however, it is not typically recognized until early childhood and adolescence when patients experience rapid growth and the severity of the depression becomes more visible. For most patients there is little resulting effect on physiology but the physical appearance can lead to psychological distress and represents a significant indication for treatment.
Clinical Features
Many patients who present with pectus excavatum are active and otherwise healthy appearing with only a visible anatomic defect as their chief complaint. Physical features include visible depression of the lower sternum with varying asymmetry of the chest wall. The deformity can be present in many different configurations with the most common being a cup-shaped concavity. The cup shape is usually well defined and deep, involving the lower end of the sternum. When the upper costal cartilages are involved, patients present with a broader and more extensive depression that is typically more severe. Patients with pectus excavatum often also have a classic posture associated with the anomaly. They are typically tall and thin with sloped ribs and rounded shoulders and a protuberant appearing abdomen. There is also a high association with scoliosis [27–29]. If symptomatic, the most common symptoms reported are nonspecific precordial chest pain, decreased exercise tolerance, frequent respiratory infections, asthma, and social anxiety regarding body image resulting in psychological distress [4].
Cardiopulmonary Features
A systolic ejection murmur is frequently observed in patients with pectus excavatum and accentuated after exercise. This murmur is likely due to the decrease in proximity between the sternum and the pulmonary artery allowing transmission of a flow murmur. EKG abnormalities can be seen due to the abnormal configuration of the chest wall that displaces the heart towards the left [30].
As a structural abnormality of the chest wall, pectus excavatum can theoretically result in abnormal respiratory mechanics as well as cardiopulmonary impairment secondary to compression of the thoracic cavity. However, there are many authors who do not believe that pectus excavatum causes any cardiopulmonary impairment. Clinically, nonetheless, there is a general consensus that following surgical repair patients demonstrate increased exercise tolerance and stamina [30].
Pulmonary Function Studies
Koumbourlis and Stolar [31] performed a comprehensive analysis of patterns of lung growth and function in 103 patients with idiopathic pectus excavatum. They found that there was a high prevalence of lower airway obstruction resulting in a pattern of obstructive lung disease rather than restrictive lung disease. They noted differences in lung function among different age groups; however, there was no evidence that the pectus deformity results in worsening lung growth or function as patients grow older.
A recent prospective multicenter study [32] of 327 pectus patients found a relatively small decrease in lung function studies preoperatively compared to normal with postsurgical improvement of approximately 6–10 %. Those with more severe anatomic depression had greater postoperative improvement in lung function. Preoperative and postoperative exercise pulmonary function tests showed that after surgery patients had a 10.2 % increase in maximum oxygen uptake and a 19 % increase in oxygen pulse. Although the differences between preoperative and postoperative pulmonary function tests were statistically significant, the authors still concluded that these changes were only modest in magnitude after repair.
Finally, a meta-analysis published in 2006 [33] of 12 studies with 313 pectus patients concluded that operative repair of pectus excavatum resulted in no statistically significant change in pulmonary function. Despite the multitude of studies published on this topic, there is still no consensus on the impact of the deformity on any objective measure of pulmonary physiology.
Cardiovascular Studies
In patients with pectus excavatum, the posterior depression of the sternum can result in an anterior depression of the right ventricle and displacement of the heart to the left [30]. Studies with small sample sizes suggest that patients with this compression have decreased stroke volume and cardiac output compared to normal controls and are limited in their ability to increase stroke volume with exercise [34]. The incidence of mitral valve prolapse is higher in pectus patients (17–65 %) as compared to the normal pediatric population (1 %). This is theorized to be the result of cardiac compression as postoperative studies suggest that up to 50 % of mitral valve prolapse resolves after pectus repair [35, 36]. Dysarrythmias such as first degree heart block, right bundle branch block, and Wolff–Parkinson–White are also common with an incidence of 16 % in the pectus excavatum population [7].
A meta-analysis published in 2006 [37] examined pre- and postoperative cardiovascular function in 169 pectus patients. The authors concluded based on the eight studies that met their eligibility criteria that on average, quantitative measures of cardiovascular function improved by greater than one half standard deviation following surgical repair. Controversy, however, remains, as a subsequent meta-analysis published in 2007 [38] reviewed the same literature and concluded that there was no reliable documentation of improvement in cardiac function after pectus repair.
Initial Assessment
The evaluation of a patient with pectus excavatum, like any other chest wall deformity, begins with a thorough history and physical exam. A careful assessment of the severity of the defect, symptomatology, and potential for limitations on cardiac and pulmonary physiology should be considered. Specifically, attention should be paid towards exercise tolerance, pain along the sternal border and lower costal margins, and psychological distress from body image issues. On physical exam, the depth of the defect and evidence of rotation should be documented [21].
Imaging studies can further assist in anatomic assessment and documentation of chest wall dimensions. These tests are particularly valuable for preoperative planning. There have been studies advocating that a routine chest radiograph be the sole imaging required for preoperative assessment [39]; however, the majority of patients undergo computed tomography (CT) scan of the chest. magnetic resonance imaging (MRI) may be used in patients where radiation exposure is a significant concern; however, CT is typically considered a more favorable imaging modality given its improved visualization of bony detail compared to MRI [7].
There are a variety of scoring indices to assess the severity of sternal depression, but the most widely accepted is the Haller index. The Haller index is calculated radiographically by dividing the transverse breadth of the chest by the narrowest sternovertebral distance. A Haller index greater than 3.25 is considered to be indicative of a severe defect [40] (Fig. 2.2).
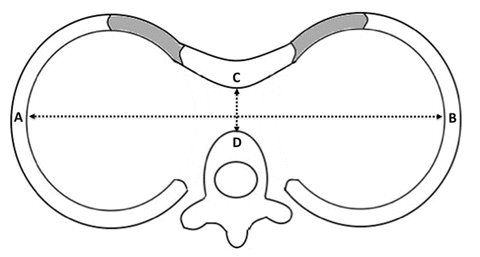

Fig. 2.2
Haller index
Almost all patients undergo pulmonary function testing prior to operative repair; however, normal results do not preclude operative intervention. Patients who have a history of palpitations should have a 24-h electrocardiogram looking for arrhythmias as well as an echocardiogram to evaluate for possible mitral valve prolapse.
Indications for Operative Repair
Factors that determine operative candidacy include anatomic changes, physiologic limitations, and psychological distress. The indications for repair used by most centers are subjective exercise intolerance, abnormal pulmonary function testing, abnormal echocardiogram, exercise-induced asthma, body image issues, and a Haller index > 3.25.
Nonoperative Management
Exercise Programs
The large majority of patients who present to referral centers are diagnosed with mild or moderate deformities and do not meet criteria for surgical intervention. These patients are typically started on exercise and posture programs. This strategy is designed to strengthen cardiopulmonary function and improve posture to allow for increased chest wall expansion as the classic pectus posture can contribute to progression of the deformity. Daily breathing and posture exercises are prescribed and patients are encouraged to participate in aerobic activities and team sports to avoid a sedentary lifestyle, which can worsen symptoms. These patients are reevaluated annually to monitor compliance and progression [41].
Sternal Suction Device
Several case series in Europe using sternal suction devices applied to the anterior chest wall have reported success in managing patients with mild deformities. The device is used daily for 12–15 months and preliminary studies demonstrate correction of the sternal depression at approximately 1 cm per month. Long-term outcomes with the device, however, are still pending [42–45].
Operative Management
Open Repair (Modified Ravitch Repair )
The open approach requires a transverse inframammary skin incision at the level of the deepest portion of the sternal defect. Skin and muscle flaps are raised elevating the pectoralis muscles to expose the entire length of the affected portion of the sternum and bilateral costal cartilages. Subperichondrial resection of all abnormal costal cartilages is performed from the union with the rib laterally to the chondrosternal junction medially via an incision along the anterior surface of the perichondrium and dissection in the avascular plane between the costal cartilage and the surrounding perichondrium. Care must be taken to preserve the perichondrium to avoid devascularization. The xiphoid is then mobilized creating a substernal plane sweeping the pleura and pericardium off their attachments to the posterior sternum. A wedge osteotomy is created to neutralize the posterior depression of the sternum. A retrosternal bar or cartilage tripods are inserted to support the sternum. The incision is then closed and retrosternal and subcutaneous chest drains are typically left in place and removed 2–3 days later [34, 46].
Further modifications to the Ravitch procedure have been described and performed at select centers. The Leonard modification uses a retrosternal wire fixated to an external brace worn for 3 months as sternal support rather than a retrosternal bar [47] and the Robicsek modification stabilizes the sternum using Marlex mesh fixed to the cartilage remnants [48] (Fig. 2.3).
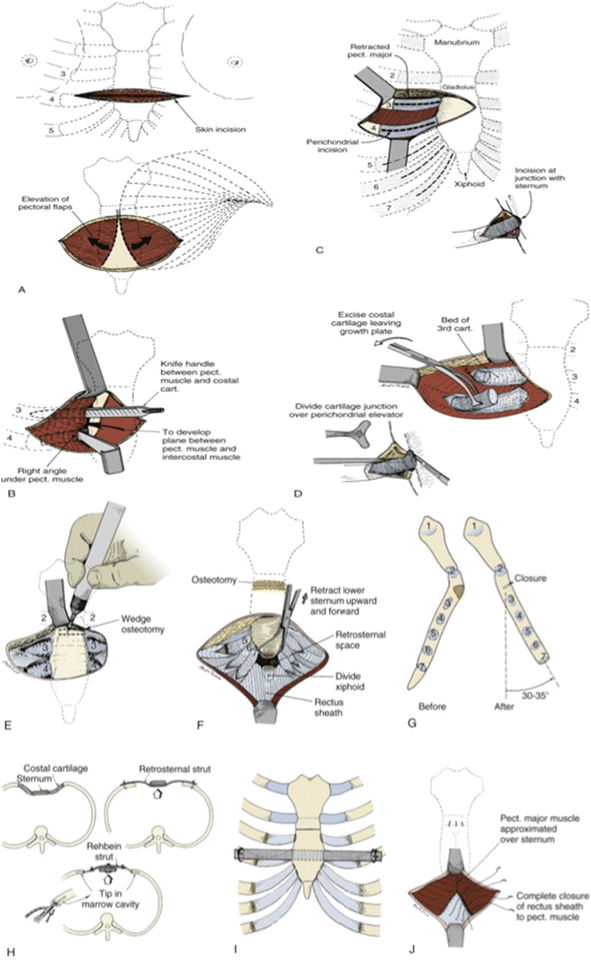

Fig. 2.3
Ravitch repair
Nuss Procedure
The minimally invasive approach described by Nuss involves surgically placing a convex steel bar underneath the sternum through small bilateral incisions. The bar is kept in place for 2–4 years allowing the sternum to reposition and remodel the deformed cartilage and rotate into a neutral position prior to removal. The original Nuss procedure has since been modified to use thoracoscopic guidance to visualize dissection of the transthoracic retrosternal plane for bar placement and to fixate the support bar in place with pericostal suturing to minimize postoperative displacement [34]. Bilateral thoracoscopic guidance or a subxiphoid incision can also help guide placement of the bar. The Nuss Procedure has become the initial procedure of choice for many pediatric surgeons who believe it to be less radical with better cosmetic results [49–52] (Fig. 2.4).
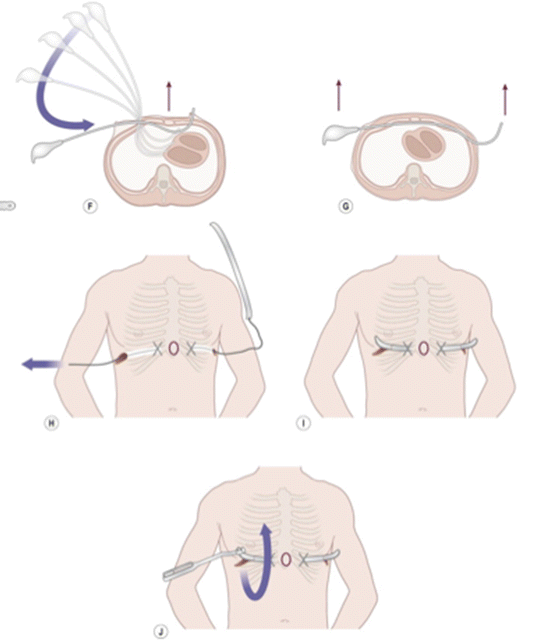

Fig. 2.4
Nuss repair
Complications
Pectus excavatum repair is generally well tolerated by patients with low rates of morbidity and mortality. A recent systematic review performed by Johnson et al. demonstrated that complication rates are similar for both procedures; however, to date, there is yet to be a randomized control trial looking at outcome differences between the two surgical approaches. Thus, the choice of operative procedure is primarily dependent on patient, surgeon, and institutional preference [15, 53–55].
The most common complications following pectus repair are bar-related events after the Nuss procedure such as displacement requiring reoperation (5.7–12 %) and pneumothorax (2–3.5 %). Wound infections (1.4–2.2 %) and pulmonary complications (2 %) such as effusions, atelectasis, and pneumonias also can occur but at less frequent rates. A recent analysis of patients undergoing the Nuss procedure using the NSQIP-P database reported a morbidity rate of 3.8 %, readmission rate of 3.8 %, and surgical site infection rate of 0.4 % [51, 52, 55–58]. Other rare but notable complications following the Nuss procedure include case reports of fatal or near-fatal hemorrhage during bar removal due to laceration of the pulmonary vessel or erosion into the sternum or aorta, or from bar rotation and erosion into the internal mammary artery [59–63]. Cases of cardiac tamponade and shock due to strut rotation and erosion to the aorta, bilateral sternoclavicular dislocation, and thoracic outlet obstruction have also been reported [62, 64, 65].
A rare but dreaded complication of the open pectus excavatum repair is acquired Jeune Syndrome (asphyxiating thoracic chondrodystrophy). In 1996, Haller et al. [66] first described 12 children who presented with severe restrictive cardiopulmonary symptoms due to a permanent impairment of normal chest wall growth after undergoing a Ravitch repair . The etiology is not completely clear; however, it is hypothesized that this life-threatening complication is the result of extensive and overzealous resection of the deformed cartilage with possible devascularization of the ossification center of the sternum. Early age of initial repair (less than 4 years old) may also increase the risk of this complication. Pulmonary function tests in these patients demonstrate a marked decreased FVC of 30–50 % and FEV1 of 30–60 %. Treatment is complex and requires re-excision of all substernal cartilage and using rib grafts or substernal support systems to reexpand the compressed chest cavity [67, 68].
Outcomes
The majority of patients report excellent patient satisfaction regardless of operative approach type. The psychological benefits have long been well documented in the literature [69]. A large multicenter trial by Kelly et al. [70] demonstrated significant improvements in patient’s body image difficulties and subjective limitations in physical activity after pectus repair. Ninety-seven percent of patients were satisfied with the postoperative cosmetic results.
Historically, recurrence rates prior to the introduction of the Nuss procedure ranged from 2 to 37 % [52]. More recent studies quote rates between 1.4 and 8.5 % [71, 72]. It was previously thought that the incidence of recurrence is higher in patients who are younger with connective tissue disorders or those who have had previous repairs [73]. However, Sacco-Casamassima et al. [71] did not find any significant patient or procedural factor predictive of recurrence in their series of 85 patients with recurrent pectus excavatum.
Other Operative Interventions
Prosthetic Inserts
Small case series in Europe and South America [74–77] have been reported treating pectus patients who present with solely cosmetic issues with custom-made silicone prosthetic implants to improve the outward appearance of the chest without changing the sternal shape. The procedure is generally well tolerated with satisfactory cosmetic outcomes. The most common complication reported is seroma formation in up to 30 % of patients [77]; however, no long-term studies have been reported.
Magnetic Mini-Mover Procedure (3MP)
The magnetic mini-mover procedure [78–80] was designed to use magnetic force to gradually remodel the pectus excavatum deformity . Rather than a large magnitude of force applied at once, a magnetic field is used to apply a small amount of force over an extended period of time. This persistent gentle force theoretically allows the sternum to remold slowly and avoids the significant pain associated with traditional surgical procedures. A disc magnet is inserted surgically through a small subxiphoid incision and secured onto the sternum. A custom-made orthotic brace houses an external magnet and is fitted to the patient’s chest. Patients wear the brace from 18 to 24 months until the internal magnet is subsequently removed. The initial pilot study did not result in a significant change in Haller index in their ten patients at 1 year of follow up, but there was a trend towards improvement in a subset of younger patients under 14. Further analysis demonstrated that the device proved to be safe and cost efficient. Currently, the magnetic mini-mover is in phase 3 multicenter trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


