Fig. 3.1
Axial diagram of magnetic mini-mover procedure (3MP)
The internal, implanted magnet (Magnimplant) consists of 1½-in. diameter, 3/16-in. thick neodymium–iron–boron magnet backed by a 1/16-in. ferromagnetic plate (to focus the magnetic field in the direction of the brace), fully encased in a low-profile titanium shell (Fig. 3.2a). The device is positioned on the front of the sternum and secured to a titanium disk back plate on the posterior sternum. In the initial design, the anterior magnet casing included a threaded post on the posterior surface, which was inserted through a hole in the depressed portion of the sternum and screwed securely into a threaded stem on a titanium disk back plate. When treatment is completed, the device is removed in an outpatient procedure.
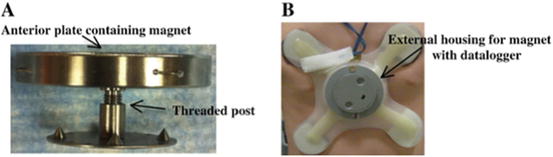

Fig. 3.2
3MP device. (a) The Magnimplant consists of a neodymium–iron–boron rare-earth disc magnet (1.5 in. diameter × 0.1875 in. thick) and a ferromagnetic focusing plate encapsulated in a low-profile titanium shell that is positioned on the front of the sternum and is secured to the sternum by screwing it into the threaded stem of a titanium disk back plate. (b) The Magnatract is a custom-made, external orthotic brace with a housing unit for a second rare-earth magnet. The brace is held in place using the attractive force between the coupled internal and external magnets, and the force exerted on the sternum is adjustable
The external device (Magnatract) is a custom-made orthotic device made of polypropylene that is molded to each patient’s chest wall (Fig. 3.2b). The Magnatract houses a second rare earth magnet that is held onto the patient’s chest wall by its attraction to the implanted magnet. The position of the magnet within the brace is adjustable, so that the strength of the magnetic force can be regulated, allowing patient-specific alterations in force and orientation. The external device also includes sensors, which measure and record the temperature and the force generated when the magnet is exposed to the magnetic implant. This sensor is wired to a custom-designed microprocessor and data logger, which records the force and temperature every 10 min. Using these data, the patient and clinician can track how long the brace is worn and how much force the magnets are exerting.
Development and Preclinical Testing
To demonstrate that the 3MP could apply enough force to reform the costal cartilages, the device was implanted in human skeletons to test the variation of force produced by attraction of the coupled magnets at various distances. The force generated on the internal magnet (and therefore the sternum) is 4.45 kg when the magnets are 1 cm apart. As the external magnet is adjusted further away, the outward force decreases (Fig. 3.3). The force necessary to move the chest wall 1 cm in an awake child is approximately 2.5–5.0 kg, varying with age and sex [1]. In contrast to traditional corrective procedures, which must move the chest wall a large distance at a single time and thus require a large amount of force (up to 23.4 kg), the 3MP must only apply enough force to stimulate the reformation of the abnormal cartilages. This stimulus can then be continuously applied over a period of months. The outward force on the sternum generated by the two-magnet system, 4.45 kg when the magnets are 1 cm apart, is in the range required to move the sternum 1 cm in an awake child and therefore is in a range capable of producing a gradual remodeling of the chest wall.
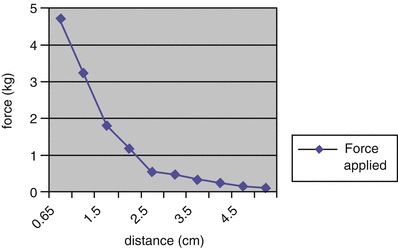

Fig. 3.3
Measurements of the strength and force generated by various distances of internal and external magnets
A significant consideration during development of the device was the safety of long-term exposure to magnetic fields from an implanted magnet, especially one placed so near the heart. To determine the magnetic field strength at the surface of the heart generated by the device, a magnetic field map was drawn with the magnets at varying distances from 1 to 10 cm apart. When the magnets were 1 cm apart, the maximum field strength reaching the undersurface of the sternum was 0.04 T. From studies investigating the risk of magnetic resonance imaging on human safety, it is accepted that there is no detectable effect on cardiac performance or hemodynamics from exposure of magnetic fields up to 1.5 T [2, 3]. Moreover, an interesting large animal model exists in the prevention of Bovine Hardware disease with “cow magnets.” These magnets are placed in one of the bovine stomachs to collect ingested wires, nails, and other metal that would otherwise cause trauma or obstruction more distally in the GI tract. The magnets, of a similar strength and at a similar distance from the heart as the 3MP, remain in place for the animal’s entire life with no ill effect [4].
Another early safety concern was whether the magnetic field generated by the device could pose a risk to others in close contact with the patient, or interfere with external devices. To decrease this risk, the Magnatract brace was covered with a thin ferromagnetic shield to decrease the magnetic field outward from the brace. Using the shield, the highest field strength at the outer surface of the orthosis decreased from 150 to 10 G [5].
Phase I Clinical Trial
Study Design
Following preclinical testing, we obtained an Investigational Device Exemption (G050196/A002) to proceed with trial in patients. Funded by a Food and Drug Administration (FDA) Office of Orphan Products grant (R01FD003341), a first-in-human trial was conducted to test the safety and proof of concept of the procedure. Ten otherwise healthy patients between 8 and 14 years of age with a pectus severity index greater than 3.5 were enrolled in the single-institution pilot study. After implantation of the Magnimplant device, patients wore their custom-fitted Magnatract external brace for the 18-month duration of the trial. Patients were followed with monthly wound checks and chest X-rays to monitor for skin changes and device integrity, respectively, and the pectus severity index (PSI) was recalculated over the course of treatment. Preoperative and post-implant removal chest CT scans were obtained to assess overall improvement in PSI. Electrocardiograms (EKGs) were obtained pre-implant, 1-month post-implant, and 1-month post-explant to monitor for any effect on cardiac electrical function.
Safety
Seven boys and three girls with mean age 12.7 (range 8–14) years participated in the pilot trial. Patient data are summarized in Table 3.1. From sensor data , the average brace wear time was 16 h per day and generally increased over the course of the study. None of the patients developed EKG changes or had any clinical signs of cardiac effects. There were no incidents of permanent skin damage or discoloration from wearing the external orthotic device. One patient experienced some mild skin erythema, but this resolved with brace reconfiguration. There were no infections from device implantation or chronic use, but three patients had a postoperative wound infection following implant removal, including one patient who required hospital admission for intravenous antibiotics and wound exploration for possible osteomyelitis, which proved negative. One patient developed a pericardial effusion 16 months after implantation, which required urgent pericardiocentesis. On extensive work-up, no evidence on imaging, echocardiogram, blood, or skin tests demonstrated an association with the implanted magnet, and it was left in place. No recurrent effusion developed, and the evaluating consultants concluded the effusion was likely viral in origin.
Table 3.1
Data of mean compliance per 24 h and pectus severity index pretreatment and posttreatment
Subject | Age at implant, years | Sex | Total months in study | Mean compliance, % | Pectus severity index at enrollment | Final pectus severity index
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|