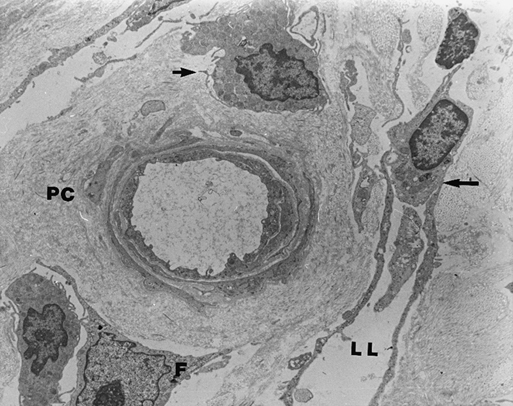
Once leukocytes migrate to the extracellular space, they localize around capillaries and postcapillary venules. The perivascular space is surrounded by extracellular matrix (ECM) proteins. Adjacent to this perivascular cuff and throughout the dermal interstitium is an intense and disorganized collagen deposition. This perivascular cuff and the accompanying collagen deposition are the sine qua non of the dermal microcirculation in CVI patients (Figure 1). The cuff is a ring of ECM proteins consisting of collagens type I and III, fibronectin, vitronectin, laminin, and tenascin. The cuff was once thought to be a barrier to oxygen and nutrient diffusion. Evidence now suggests, however, that cuff formation is an attempt to maintain vascular architecture in response to increased mechanical load.
Pathophysiology of Venous Varicosities and Venous Ulcers
Extracellular Matrix Alterations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
