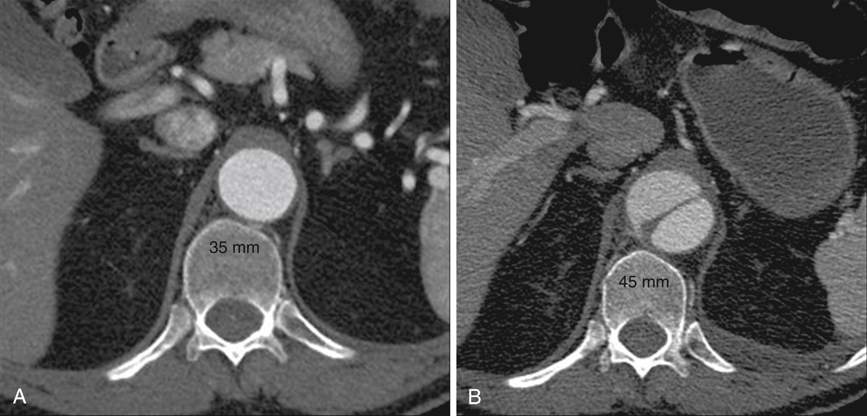
Anecdotal review of computed tomography (CT) scans in patients who fortuitously had a study at the time of acute dissection as well as one shortly before the dissection show that the dissected aorta can acutely grow to a diameter 25% greater than its baseline (Figure 1). The anatomy of the two lumens explains this phenomenon. The normal aorta expands as a result of blood pressure until wall tension generated by elastic recoil of the mural elastin and collagen balances blood pressure. In aortic dissection, the dissection flap typically contains the intima and two thirds of the media, and the outer wall of the false lumen contains the remaining one third of the media and the adventitia. Being thinner and less elastic than the outer wall of the undissected aorta, the outer wall of the false lumen must expand to a larger diameter to generate, at a given blood pressure, the same wall tension. The dissection flap, which lies between isobaric lumens, has been released from transmural pressure and therefore undergoes radial elastic collapse. Thus false lumen dilation and true lumen collapse are to be expected, given the structural characteristics of the aortic wall.
Pathophysiology of Aortic Dissection
Early Anatomic Changes in Aortic Dissection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
