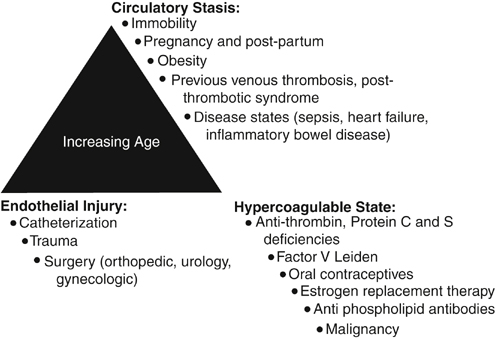
Daniel D. Myers, Jr., Evelyn M. Shea and Thomas W. Wakefield The factors that could lead to or interact with one another to lead to VTE include blood stasis, endothelial injury, and blood hypercoagulability. Of these three factors, vein wall endothelial injury triggers a local inflammatory response, which promotes a prothrombotic state driven by tissue factor, adhesion molecules, hemostatic factors, and proinflammatory cytokines that support acute VTE. In 1974, it was first hypothesized that vascular inflammation and thrombosis are interrelated. The original hypothesis suggested that prothrombotic factors lead to the activation of leukocytes and platelets. This process promotes thrombus amplification via adherence and layering of the activated platelets and leukocytes. Risk factors for acute VTE include increasing age, pregnancy, prolonged periods of immobility, estrogen therapy, surgery and trauma, malignancies, hypercoagulable states, cardiovascular disease (myocardial infarction, congestive heart failure), inflammatory diseases, obesity, and for VTE recurrence male gender (Figure 1).
Pathophysiology of Acute Venous Thrombosis
Risk Factors for Acute Venous Thromboembolism
Thoracic Key
Fastest Thoracic Insight Engine