PATENT DUCTUS ARTERIOSUS IN THE PREMATURE INFANT
Introduction
The ductus arteriosus (DA) is 1 of the 3 major shunts, essential for fetal survival in utero. The other 2 shunts, ductus venosus (DV) and foramen ovale (FO), are equally essential for fetal survival in utero. The DA is a remnant of the left sixth aortic arch, and it connects the main pulmonary artery (MPA) to the descending aorta (DAo) beyond the left subclavian artery. In term infants, it is about 1.25 cm in length, and 0.5 to 0.7 cm in diameter. In utero, only 5% to 10% of the right ventricular output passes through the lungs and the rest (90%) is shunted across the ductus into the DAo.1 The high pulmonary vascular resistance (PVR) and low systemic resistance (due to umbilical-placental circulation) in utero contributes to this right-to-left shunt. Premature closure of the DA, either spontaneously or from maternal intake of nonsteroidal antiinflammatory drugs (NSAIDs), is associated with increased PVR, early right heart failure, fetal hydrops and death in utero.2 In healthy newborns, all the 3 fetal shunts do cease to function soon after birth and close during the first 2 to 3 days.3 The closing of these fetal channels have been closely related to (1) fall in PVR and increase in systemic resistance; (2) decrease in prostaglandin (PG) levels, especially PGE1 and PGI2; and (3) rising oxygen tension. Closure of the ductus in preterm infants, as discussed below, is delayed and differs significantly due to developmental differences in smooth muscle and many other factors related to prematurity. Earlier reports from 1970 to 1990 have shown that persistence of DA with left-to-right shunt is associated with significant morbidity such as chronic lung disease (CLD), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC) and mortality.4,5 However, more recent studies on early closure of patent ductus arteriosus (PDA) with NSAIDs or by surgery have failed to show any such cause and effect relationship.6–8
For most preterm infants affected by a PDA, the hemodynamic effects are not immediately life threatening and can be controlled medically without intervention to close the ductus. Presently, neonatologists have a dilemma as to whether to treat, when to treat and how to treat PDA in extremely low birth weight infants. With more mothers receiving antenatal steroids and neonates receiving better Neonatal Intensive Care Unit (NICU) care, more preterm infants are surviving and are diagnosed with PDA. In this chapter, the focus is on the incidence, clinical presentation, underlying hemodynamic changes, diagnosis, management options and long-term outcome.
Incidence
In 1959, Burnard, in his first description of PDA noted that the incidence of PDA is higher in preterm infants.9 Since then, several studies have confirmed these findings.10,11 In term infants (>37 weeks gestation), the incidence is 0.05% (1 in 2000; range: 0.03%–0.08%).12 In preterm infants the incidence varies inversely with gestational age. In infants <1000 g, the incidence has varied from 34% to 70%.13 Another study in infants requiring ventilator support and surviving beyond 3 days reported higher incidence of PDA: 78% at 24 to 25 weeks, 40% to 45% at 26–29 weeks, and 17% at 32 to 33 weeks.14 Antenatal steroid use during that period was much lower than today. Incidence of significant PDA is also higher and occurs earlier in preterm infants with intrauterine growth restriction (60%) compared to appropriately grown infants (15%).15 Other factors that increase PDA incidence are (1) infants exposed to antenatal magnesium sulfate16; (2) postnatal infection17; (3) higher fluid intake during the first week18; (4) infants requiring phototherapy19; (5) presence of severe respiratory distress20 and (6) genetic predisposition – TFAP2 B+.21 Whether recent management changes such as accepting lower threshold oxygen saturation in very low gestational age (VLGA) infants has any impact on the incidence of DA is not known. Table 39.1 below shows the incidence of PDA, number treated with indomethacin or ibuprofen and the presence or absence of bronchopulmonary dysplasia (BPD), according to gestational age at Children’s Hospital of Wisconsin for the year 2012.
Table 39.1. Incidence of PDA in Very Low Birth Weight infants at Children’s Hospital of Wisconsin
| Gestational Age (weeks) | N | Survived | PDA (%) | Medically Treated | Ligated | BPD With PDA | BPD No PDA |
| 23–27 | 53 | 46 | 27/53 (51%) | 26/27 (96%) | 10/27 (37%) | 22/27 (81.5%) | 11/22 (50%) |
| 28–32 | 126 | 126 | 6/126 (4.7%) | 4/6 (67%) | 0 | 5/6 (83%) | 9/120 (7.5%) |
BPD: Bronchopulmonary dysplasia
Pathophysiology of Ductal Closure
In utero DA shunts >90% of combined ventricular output away from lungs. After birth with the expansion of lungs, fall in PVR and rise in systemic resistance the shunt ceases or becomes bidirectional at least in the transitional period. In term infants, the functional closure is complete by 3 days.3 Pulsed Doppler studies in normal, healthy newborns showed that functional closure of DA occurs in 50% by 24 hours, 90% by 48 hours, and 100% by 72 hours after birth.22 Unlike the aorta (Ao) and pulmonary artery (PA), the medial musculature of DA has more longitudinal and spirally arranged smooth muscle fibers and concentric layers of elastic fibers. The rising oxygen tension inhibits voltage-dependent potassium channels in smooth muscle that leads to calcium influx and ductal constriction.23 Subsequent fibrotic closure of DA takes 3 to 4 weeks and the resulting fibrotic band is called ligamentum arteriosum. This oxygen-induced ductal constriction is maturation dependent and is mediated by (1) ductal smooth muscle cell depolarization and calcium influx; (2) rise in vasoconstrictor endothelin-124; and (3) production of reactive oxygen species at the mitochondrial level, which can activate myosin light chain phosphorylation via Rho/Rho-kinase pathways.25 Ductal constriction leads to hypoxia, ischemia, and endothelial injury, with a resultant decrease in PGE2 and nitric oxide (NO) production and an increased production of cytokines26 and growth factors such as vascular endothelial cell growth factor (VEGF), which helps in ductal remodeling.27 Echtler et al, using a mouse model, showed that recruitment of platelets to the luminal wall is essential for luminal remodeling of ductus soon after birth.28 Two recent retrospective studies in preterm infants showed that thrombocytopenia in the first few days is associated with significant PDA and a higher failure rate of ductal closure with indomethacin.28–30 In preterm infants, the ductal wall is thinner, the lumen is larger, and the postnatal constriction does not obliterate the lumen and the ductal wall does not become ischemic, in a manner that happens in the term infants. These findings suggest that ductal closure in preterm infants is a complex process with multiple players whose role is yet to be fully understood. One recent experimental study in baboons and another preliminary study in preterm infants <28 weeks have shown that indomethacin when given along with NO synthase inhibitor [N(G)-monomethyl-L-arginine (L-NMMA)] produces better ductal constriction than indomethacin alone.31,32
Hemodynamic Changes in PDA
The magnitude of the left-to-right shunt through PDA depends on the size of the ductus and in the large ductus, the pressure (vascular resistance) difference between systemic and pulmonary circulations. The higher the difference, the larger the size of the left-to-right shunt. Higher pulmonary blood flow results in higher venous return to left atrium (LA) and to left ventricle (LV; high preload). Less distensible and less compliant LV of premature infant initially responds to this increased preload by increasing stroke volume. With large left-to-right shunts (>50% LV output) systemic blood flow can be affected resulting in distal organ hypoperfusion, manifesting hypotension, renal dysfunction and metabolic acidosis. In addition, large left-to-right shunt results in increased pulmonary blood flow and increased hydrostatic pressure in pulmonary capillaries. This combined with a low oncotic pressure33 results in increased filtration of protein and fluid into the interstitial and alveolar space causing pulmonary edema and even pulmonary hemorrhage. Earlier studies on pulmonary mechanics have reported decreased pulmonary compliance with hemodynamically significant PDA (hsPDA) and its subsequent improvement with closure of ductus.34 In most infants with hsPDA, it may take 7 to 10 days to develop pulmonary edema and signs of congestive heart failure (CHF). If the CHF is not treated adequately, it can lead to systemic hypotension and hypoperfusion, acidosis, and even death. Increased blood flow to lungs not only affects pulmonary mechanics, but also lung development. Experimental studies in premature baboons with open PDA have shown delayed alveolar development and decreased alveolar surface area compared to the group with early ductal closure using ibuprofen.35
Diagnosis of PDA in Preterm Infants
In preterm infants, it is the presence of clinical signs or the radiological findings that alert the neonatologists to the patency of DA with left-to-right shunt. Over the last 20 years, newer diagnostic markers have been used to assess hsPDA.
Clinical Findings
Clinical findings in preterm infants may depend on the severity of illness, and shunt size. The size of the lumen (minimal ductal diameter), the difference between PVR and systemic resistance, usually determines the amount of blood shunted across ductus. In extremely preterm infants (<1000 g) treated with prophylactic surfactant in the delivery room, a loud murmur may appear early because of rapid decrease in PVR. Presence of continuous murmur is considered classic for PDA in older infants and children but is rarely heard in preterm infants. Audible murmur may be present in 20% to 50% of preterm infants. Other manifestations of PDA include wide pulse pressure, decrease in both systolic and diastolic pressures, hyperactive precordium and bounding peripheral pulses. However, none of these findings have high sensitivity or specificity.36 Moreover in the first few days of life, these findings may vary hour-to-hour and day-to-day depending on arterial oxygen tension and fluid intake. Other clinical manifestations may depend on the extent of systemic blood flow compromise, for example, decreased urine output, hypotension and metabolic acidosis.
Delta Perfusion Index (PI)
A recent report investigated the usefulness of differential PI as a diagnostic tool to identify hsPDA. The study used signal extraction technology using a Masimo Radical sensor. The hypothesis is based on the assumption that hsPDA will decrease perfusion to lower extremity thus creating a differential perfusion between upper and lower extremity. Pooled data from a small group of preterm infants (N = 30) with or without hsPDA showed that a delta PI cutoff value of 1.05% had a sensitivity, specificity, positive and negative predictive value of 66.7%, 100%, 100% and 86.4%, respectively. The investigators concluded that in the presence of clinical signs of PDA and a delta PI of >1.05% diagnosis of hsPDA should be considered37 and repeated echocardiographic studies may be avoided. When confirmed by further studies, this technique can be of some help in places where echocardiography is not readily available.
Radiological Findings
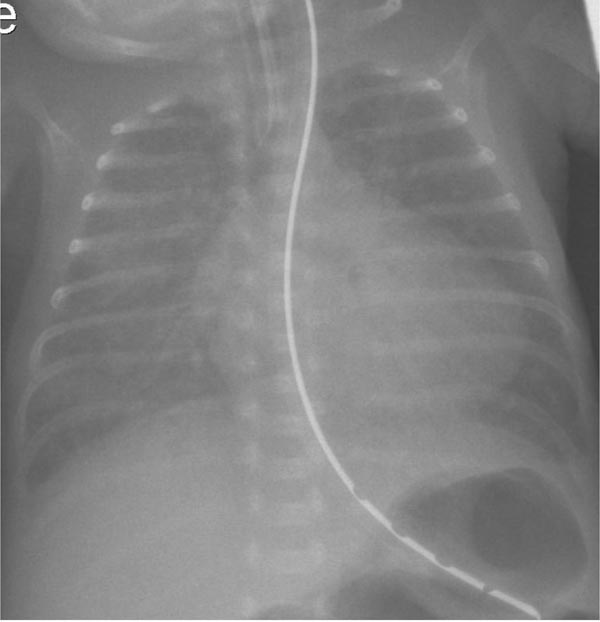
Prior to the routine use of antenatal steroids and postnatal use of surfactant, classic radiologic findings of respiratory distress syndrome included congested, hazy or ground-glass lung fields; this was resolved by day 3 with the onset of diuresis. The reopacification of lung fields along with the presence of clinical signs indicated significant left-to-right shunt through DA. Cardiomegaly with the apex of the heart pointing down suggesting left ventricular dilatation may be present with a large left-to-right shunt (Figure 39.1). One report showed cardiomegaly and presence of pulmonary plethora in 30% of infants with hsPDA.38 Other radiological findings include elevation of left main stem bronchus from dilated LA (pancaking LA). A recent study reported that a carinal angle of >90° had 85% sensitivity and specificity for the presence of PDA.39 This may be more useful in places where echocardiogram is not readily available.
Figure 39.1. Chest roentgenogram of a preterm infant (birth weight of 400 g, gestational age 25 4/7 weeks) with PDA showing cardiomegaly and interstitial edema. Image obtained on day 22 of life. Echocardiogram showed moderate PDA.
Two-Dimensional (2D) Echocardiography with Color Doppler Flow Mapping
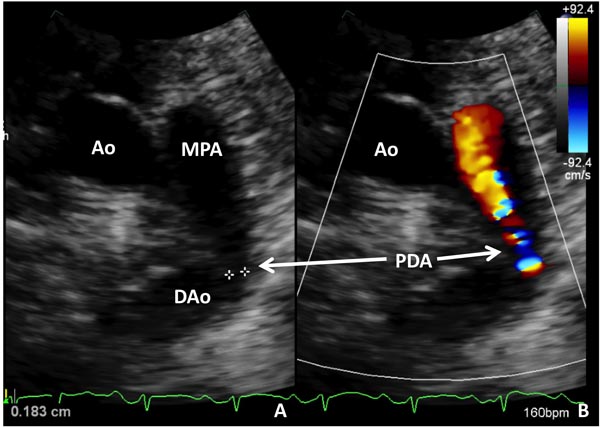
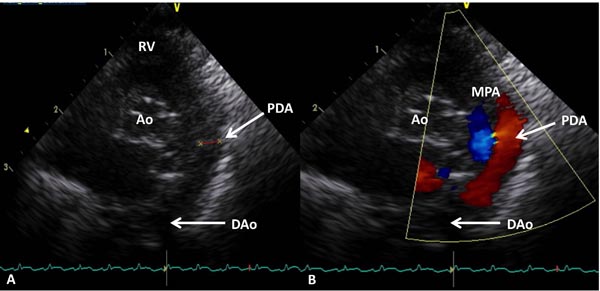
Among all the markers used for the diagnosis of PDA, 2D echocardiography with color flow Doppler has been considered as the gold standard or criterion standard.40 Today, in most western countries this is the standard of care. Advantage of echocardiography is that the test is done at bedside and the results are available quickly in most cases. Usually, pediatric cardiologists read the echocardiogram in most centers. 2D echocardiogram with color Doppler can provide the following information: (1) patency of the ductus; (2) direction of the shunt; (3) left ventricular size and contractility; (4) pulmonary to systemic blood flow ratio (volume of ductal shunt); (5) ductal diameter by 2D and color Doppler and (6) presence and quantitation of retrograde diastolic flow in the DAo. One recent retrospective report of 29 infants <29 weeks gestation showed that a PDA diameter >1.5 mm assessed by early color Doppler (between 6 and 48 hours) predicted the development of symptomatic PDA. It had the sensitivity and specificity of 91% and 100%, respectively. In this study, 29 infants had early color Doppler assessment and 20/29 had significant PDA. None of the infants received prophylactic or rescue treatment with cyclooxygenase (COX) inhibitors during the study period.41 Examples of small (Figure 39.2) and large (Figure 39.3) PDAs are shown. Readers interested in echocardiographic diagnosis of PDA can refer to the 2 other recent reviews by Evans and coworkers42,43 as well as a review on echocardiography in Chapter 11.
Figure 39.2. Selected video frames from precordial short-axis view demonstrating 2D (A) and color flow Doppler (B) images of a small PDA (arrows) with left-to-right shunt. Ao, aorta; DAo, descending aorta; MPA, main pulmonary artery.
Figure 39.3. Selected video frames from precordial short-axis view demonstrating 2D (A) and color flow Doppler (B) images of a large PDA (arrows) with left-to-right shunt. Ao, aorta; DAo, descending aorta; MPA, main pulmonary artery; RV, right ventricle.
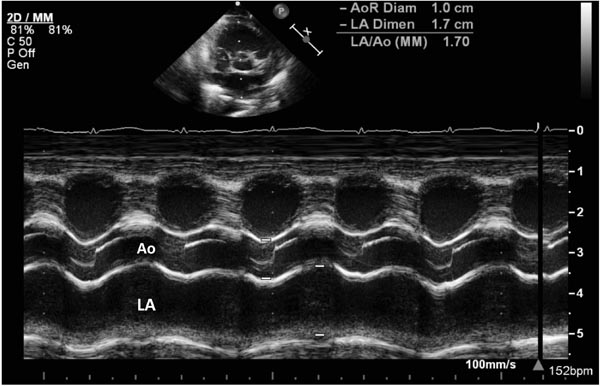
M-mode echocardiography has been in use since the early 1980s to measure the diameter of LA and aortic root ratio (LA/Ao ratio). An example is shown in Figure 39.4. Since a large left-to-right shunt via ductus increases volume load on the left side of the heart and results in left atrial dilatation. A LA/Ao ratio >1.5:1 is suggestive of moderate and ratio of >1.8–2:1 is suggestive of large hsPDA. However, the sensitivity of this method is only 52% to 71%. False positive results have been seen in babies with AV valve regurgitation, and false negatives in infants with restricted fluid intake. Assessment of LA/Ao ratio along with left-sided systolic time intervals has shown to increase the sensitivity and specificity in the diagnosis of PDA.44 A ductus is considered small when ductal diameter is 1.4 mm or less, LA:Ao ratio 1.4:1, and the diastolic flow in the DAo is antegrade, where as a diameter of 2.0 mm or more along with LA:Ao ratio >1.6 to 1.8:1 is considered large.
Figure 39.4. Selected video frame from precordial short-axis view with M-mode tracing that shows the sizes of the Ao and LA to measure LA/AO ratio. In this baby, the LA/Ao ratio was 1.7 (see insert).
Recently, McNamara and Sehgal have described a PDA staging system for preterm infants based on clinical and echocardiographic criteria in an effort to identify babies who require surgical ligation.45 The clinical staging was based on oxygenation index, need for respiratory support, inotropic agents, clinical evidence for organ dysfunction and presence of metabolic acidosis, while echocardiographic criteria included size of the ductus, LA:Ao ratio, signs LV overload, decreased, absence or reversal of diastolic flow. A large, hemodynamically significant ductus will show clinical signs of oxygenation difficulty, decreased renal or mesenteric perfusion, hypotension, and acidosis along with echocardiographic findings of ductal diameter of >3.0 mm, severe left heart overload, and reversal of end-diastolic flow in superior mesenteric, middle cerebral, or renal artery. Such a staging system sounds logical and when validated may help to identify hsPDA that require intervention.
Biomarkers in PDA
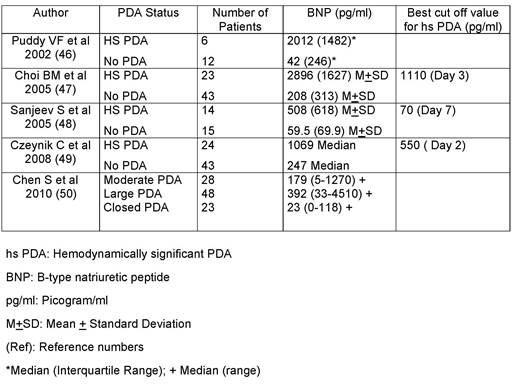
Use of serum and urinary biomarkers are increasingly used for the early diagnosis of hsPDA. Several biomarkers, namely brain natriuretic peptide [or B type natriuretic peptide (BNP)], N-terminal-pro-B type natriuretic peptide [NTproBNP] and Troponin T (TnT), have been measured in plasma of newborns. Of the 3 markers, BNP is the most studied peptide in preterm infants. BNP is released from the ventricles in response to volume load or pressure load and has been used as a marker for heart failure. BNP levels in term newborns are lower than in preterm infants. In preterm infants with PDA and left-to-right shunt, there is an excessive load on the LV, which results in LV dilatation. Thus, sequential measurements of BNP can theoretically help in identifying hsPDA. Data from the 5 studies that monitored BNP levels in preterm infants during the first week of life are shown in Table 39.2.46–50 These studies monitored BNP levels in small numbers of preterm infants and have used different cutoff levels as markers of hsPDA. Several factors may explain this marked variability. Among them, daily fluid load and magnitude of left-to-right shunt and ductal constriction in response to treatment may play a major role. Surgical ligation has been shown to decrease the BNP levels. Other biomarkers, such as TnT and NTproBNP, have also been used as a marker for hsPDA.51,52
Table 39.2. BNP Levels in Preterm Infants with PDA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree