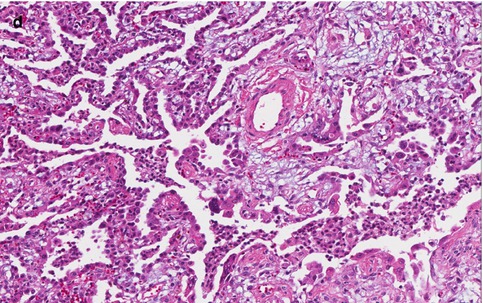
Fig. 10.1
Lung biopsy with parainfluenza infection. (a) Acute and organizing pneumonia with bronchiolitis and multiple multinucleated giant cells consistent with infection by parainfluenza virus. (b, c) Multinucleated giant cell present in inflamed bronchiole. (d) Organizing acute lung injury present in surrounding alveolated parenchyma
10.8 Diagnosis
Most infections are diagnosed clinically. The diagnosis can be confirmed either by isolation and identification of the virus in cell culture, direct detection of viral antigen or nucleic acid by immunoassays and PCR, or by serologic tests. The most reliable method for diagnosis is isolation of the virus in tissue culture. Throat swabs, nasopharyngeal swabs, nasal washes, and nasal aspiration are acceptable specimens (Frayha et al. 1989; Hammitt et al. 2011; Chan et al. 2008). Although it can be hard to obtain, sampling of the lower respiratory tract by techniques such as lavage may be necessary in lower respiratory tract infection in immunocompromised patients or transplant recipients.
Methods for direct detection of the viral antigen in respiratory secretions include immunofluorescence, enzyme immunoassay, fluoro-immunoassays, and radioimmunoassay. These methods can be used to detect the virus in nasopharyngeal secretions, but the sensitivities of these tests vary (Hierholzer et al. 1989; Sarkkinen et al. 1981; Halonen et al. 1983). Demonstration of a four-fold or greater rise in specific IgG antibody titers in the blood is diagnostic in primary infections. However, heterotypic cross reactivity limits its usefulness in repeated infections, and infections are not always accompanied by a significant antibody response. There are multiple PCR assays that have been used widely for detection of multiple respiratory viruses, including parainfluenza viruses. Several studies demonstrate these assays to have a high specificity and sensitivity. Initially, these assays were mostly monospecific, requiring separate amplifications for each HPIV type (Echevarría et al. 1998; Coiras et al. 2004; Fan and Henrickson 1996). However, multiplex RT-PCR assays have been developed for detecting HPIV-1, HPIV-2, HPIV-3, and HPIV-4 (Eugene-Ruellan et al. 1998; Bellau-Pujol et al. 2005; Osiowy 1998).
10.9 Differential Diagnosis
Patients with croup typically present with a history of coryza and low-grade fever and then develop a classic “barking” cough associated with croup. Croup should be differentiated from epiglottitis, which is characterized by the abrupt onset of severe symptoms. Without airway control and medical management, epiglottitis may rapidly progress to respiratory obstruction and death in a matter of hours. Croup should also be differentiated from retropharyngeal abscess. The differential diagnosis of pneumonia caused by parainfluenza virus includes pneumonia caused by adenoviruses, enteroviruses, and influenza viruses as well as infections by Chlamydia trachomatis and Mycoplasma.
10.10 Prevention
Patients prone to increased morbidity and mortality include infants, immunocompromised individuals, and the elderly. Croup is most common in children younger than 6 years of age. Signs and symptoms are most severe in children younger than 3 years of age because of their smaller airways. Passively acquired maternal antibodies may play a role in protection from HPIV types 1 and 2 in the first few months of life. Standard and contact precautions with an infected patient should decrease the spread of the virus to others.
10.11 Treatment and Outcome
There is no antiviral therapy for any of the parainfluenza viruses. However, most infections are self-limited and require no treatment. Although most children with croup improve after 48 h, some cases may take longer to resolve. Monitoring for hypoxia and hypercapnia in more severely affected children with lower respiratory tract disease may be helpful. Nebulized or racemic epinephrine may be administered to severely affected hospitalized patients with croup to decrease airway obstruction (South and Williams 2012). Systemic steroid therapy in high doses has been shown to be of benefit in children with moderate to severe croup (Dobrovoljac and Geelhoed 2012; Geelhoed 2005). Inhaled steroids appear to have limited value.
10.12 Vaccine
There is no vaccine available for HPIV. First trials for inactivated HPIV vaccines started in late 1960s (Chin et al. 1969) but failed to show complete immunity to natural infection. Bovine HPIV-3 shows promising results in seronegative individuals (Karron et al. 1995). Recombinant bovine HPIV-3 has induced good antibody and protection in lab animals (Schmidt et al. 2001). A cold-adapted HPIV-3 vaccine has been developed and has demonstrated good infectivity, immunogenicity, attenuation, and stability (Belshe and Hissom 1982; Crookshanks and Belshe 1984). Further studies are warranted to produce the first licensed HPIV vaccine.
10.13 Clinicopathologic Capsule
Parainfluenza viruses have been generally disregarded as pathogens in spite of their importance in pediatric lower respiratory illness. There are five viruses in this group (HPIV-1, HPIV-2, HPIV-3, HPIV-4a, and 4b). Human HPIVs can cause severe respiratory diseases. Clinically, the disease is manifested as an upper or lower respiratory tract infection. Syncytial multinucleated giant cells in the setting of inflammation are distinctive features of parainfluenza pneumonia. HPIV-3 is the most important pathogen and most vaccine development efforts have been directed towards this virus.
References
Akizuki S, Nasu N, Setoguchi M et al (1991) Parainfluenza virus pneumonitis in an adult. Arch Pathol Lab Med 115:824–826PubMed
Chin J, Magoffin RL, Shearer LA et al (1969) Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89:449–463PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


