Hiatal hernia type
Anatomy
I
The EGJ herniates above the diaphragmatic crura, often moving transiently from the abdomen into the mediastinum
II
A portion of the stomach is herniated into the mediastinum alongside the esophagus, with the EGJ in normal (i.e., intra-abdominal) position
III
The EGJ is above the hiatus and a portion, or the entirety, of the stomach is folded alongside the esophagus
IV
An intra-abdominal organ other than the stomach is additionally herniated through the hiatus
Presenting Symptoms
Patients with PEHs commonly present with symptoms due to either intermittent obstruction or gastroesophageal reflux (GER). Obstruction is caused by a kinking of the esophagus and/or stomach and results in episodes of dysphagia, early satiety, regurgitation, nausea, vomiting, and/or chest pain. The anatomic distortion of PEHs often leads to an incompetence of normal EGJ function [6]. This in turn causes GER, with its characteristic symptom of intermittent retrosternal heartburn, which is often postprandial and exacerbated when supine. PEHs can also result in erosions of the gastric mucosa, termed “Cameron ulcers.” These ulcers can cause anemia from chronic bleeding, and their exact etiology has not been conclusively determined [7]. Friction from repeated passage of the stomach through the hiatus, increased acid exposure from stasis of gastric juices, and ischemia have all been proposed as causal mechanisms [7, 8]. Larger type III and IV hernias can additionally cause respiratory and cardiac impairment via direct compression of the lungs and heart [9].
The symptoms discussed so far are usually subacute, and patients can suffer for prolonged periods of time while being evaluated and are often incorrectly treated for more common conditions such as non-hernia-related GER, peptic ulcer disease, angina, and biliary colic. This scenario of clinical manifestation is distinct from patients who present acutely with an incarcerated PEH. Acute PEH incarceration is a life-threatening surgical emergency, as it can lead to gastric ischemia and, if not alleviated, necrosis. The classic presenting symptoms and signs of an acute incarceration are “Borchardt’s triad” of chest pain, the urge but inability to vomit, and failure of nasogastric tube passage below the diaphragm. Immediate reduction of the hernia is required to restore blood flow to the stomach, and a laparotomy or thoracotomy is often necessary to achieve this. The remainder of this chapter will address only the evaluation and management of patients with PEH in an elective setting.
Indications for Surgery
Based on the potential for gastric incarceration, it was a long accepted surgical principle that PEHs should be repaired on an elective basis when discovered, regardless of the patient’s symptoms [10, 11]. This traditional assumption was challenged by a landmark study by Stylopoulos and colleagues in 2002 [12]. The authors constructed a Markov Monte Carlo analytic model using pooled outcomes data to estimate quality of life years for patients with asymptomatic PEH, treated with either laparoscopic repair or watchful waiting. This analysis showed that watchful waiting resulted in a yearly acute incarceration rate of only 1.1 %, and was superior to surgery for 83 % of patients. Based on these findings, expectant management is now considered a reasonable option in patients with truly asymptomatic PEH. On the other hand, the presence of any symptoms related to PEH, whether due to obstruction or GER, is considered an indication for laparoscopic repair, as long as the patient is of reasonable operative risk.
Preoperative Evaluation
In addition to a thorough history and physical examination, several tests are indicated preoperatively in order to secure the diagnosis of PEH and help define the anatomy and physiology of the esophagus and stomach. Contrast esophagram, or an “upper GI study,” forms the basis for diagnosis of PEH and description of its anatomy. The location of the esophagus, EGJ, stomach, and pylorus can all be assessed. This secures the diagnosis and subclassification within hiatal hernia type and allows the surgeon to approximate the size of the hernia sac and width of the crural defect. The distance between the EGJ and hiatus can also be measured, which if >5 cm, serves as a predictor that a esophageal lengthening procedure may be required [13, 14]. The use of fluoroscopy to obtain multiple images over time allows for an assessment of esophageal function. Pooling of a contrast column within the esophagus and a delay in contrast transit through the EGJ indicate a functional obstruction as a result of the hernia. Conversely, reflux of contrast material from the stomach back into the esophagus is indicative of an incompetent EGJ resulting in GER.
Upper endoscopy is mandatory in the preoperative evaluation of patients prior to planned PEH repair. The primary purpose is to rule out a malignancy near the EGJ, which can present with the same obstructive symptoms as PEH. It is also important to check for the presence of esophagitis or gastritis, Barrett esophagus, Cameron ulcers, and/or peptic ulcer disease. It should be noted that upper endoscopy can be extremely challenging in these patients, especially those with type III PEHs, and the risk of esophageal perforation can be increased if not performed by a skilled endoscopist.
Although not universally adopted, we routinely perform an esophageal manometry study on patients being evaluated for PEH. This study is often technically difficult to perform in these patients [15], and it is often easiest to place the manometry catheter during endoscopy. The advance to high-resolution manometry is particularly useful in the setting of PEH, as the catheter does not have to be moved once it is positioned across the EGJ. Despite these challenges, it is useful to assess the peristaltic function of the esophagus preoperatively. Patients with PEH often have abnormal esophageal motility, and these impairments can improve after surgery [16]. However, in patients with complete aperistalsis on preoperative manometry or those who have weak peristalsis and dysphagia that cannot be explained by the anatomy seen on esophagram, we will tailor our operation to include a partial, rather than complete 360°, fundoplication. Additionally, high-resolution manometry can be used to measure the distance between the EGJ and diaphragmatic hiatus (i.e., distance between high-pressure zone and respiratory inversion point), which can help stratify the risk of requiring an esophageal lengthening procedure.
Although PEH can result in pathologic GER, obtaining a 24-h pH monitoring study does not add any useful information preoperatively. This is because the dissection required to perform an effective repair will likely alter the physiology of the EGJ, and patients with PEH and heartburn (i.e., who are symptomatic) should undergo surgical repair regardless of the findings of pH monitoring.
Operative Technique
Patient Positioning and Setup
Laparoscopic PEH repair is performed under general anesthesia with endotracheal intubation and full paralysis. Patients are positioned supine with legs abducted. We tuck the right arm and abduct the left arm and use a vacuum beanbag mattress to support the patient’s sides and perineum. This positioning provides stability when the table is shifted into a steep reverse Trendelenburg position and helps to prevent neuropathy during what may be a lengthy operation. Pneumatic compression stockings and a urinary catheter are placed, and patients receive appropriate antibiotic prophylaxis prior to the initial incision.
Trocar Placement
Five trocars are utilized: one for the laparoscope, two for the operating surgeon, one for the assistant, and one for a liver retractor (Fig. 13.1). We begin by placing a 10-mm trocar slightly to the left of midline and superior to the umbilicus, approximately 12–15 cm from the xiphoid process. This is typically done using a Veress technique in patients without prior upper abdominal surgery, but an open Hasson technique may be used as well. Once this trocar is inserted and the abdomen insufflated, a 30- or 45° laparoscope is inserted and an initial diagnostic laparoscopy is performed. Use of an angled laparoscope during PEH repair is essential so that unobstructed views can be obtained when working in the confined space of the hiatus and mediastinum.
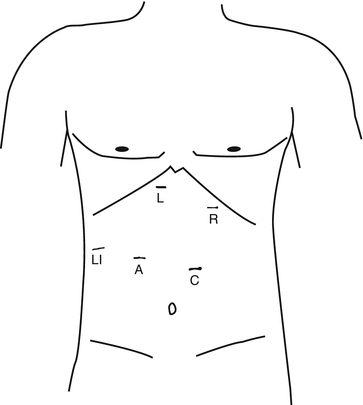

Fig. 13.1
Trocar positioning for laparoscopic PEH repair: R surgeon’s right hand instrument, L surgeon’s left hand instrument, A assistant’s instrument, LI liver retractor, C camera port (Adapted from Vaziri and Soper [17])
A 5-mm trocar for the liver retractor is then placed just below the right costal margin, approximately 15 cm from the xiphoid. We use a self-retaining retractor to elevate the left lateral segment of the liver and expose the hiatus. A 5-mm port for the assistant’s instrument is then placed in the right upper abdomen, approximately midway between the liver retractor and laparoscope ports. A common alternative is to place the assistant’s trocar in a lateral position below the left costal margin [18].
The two trocars for the operating surgeon’s instruments are then placed. The positioning of these ports is intended to create a triangulation effect, in which the two instruments enter the operative field at a 30–60° angle from either side of the laparoscopic image. The esophagus enters the abdomen through the hiatus at a right-to-left angle, so the surgeon’s two working trocars are also arranged “off center” towards the patient’s left side. For the surgeon’s right hand, a 10-mm trocar (to accommodate a curved needle) is inserted just inferior to the left costal margin, approximately 10 cm from the xiphoid process. We lastly place the surgeon’s left hand 5-mm trocar, slightly inferior and to the right of the xiphoid process. Depending on the size and anatomy of the liver, this trocar may need to be placed more inferiorly on the abdominal wall. For this reason, once the liver retractor has been secured, we test potential locations for this trocar by first passing a Veress needle through the abdominal wall to ensure that the working instrument will have a clear path to the hiatus.
Once the trocars have been placed, the patient is tilted to a steep reverse Trendelenburg position in order to shift the abdominal contents inferiorly, away from the hiatus, and to bring the patient’s upper abdomen closer to the surgeon, thereby improving ergonomics. This should be done slowly, and in coordination with the anesthesiologist, as this maneuver can significantly reduce venous return. The operating surgeon then moves to a position between the patient’s legs with the laparoscopic monitor placed directly over the head of the patient. The assistant stands to the patient’s right and the camera operator is seated in a stool to the patient’s left.
Dissection and Reduction of the Hernia Sac
A thorough diagnostic laparoscopy is then performed, focusing on delineating the hernia anatomy. This can be difficult on initial inspection, as a significant portion of the stomach may be lying in the mediastinum. Of importance to note at the onset of the operation are the positions of the pylorus, left gastric artery, spleen, and short gastric vessels, as well as the width of the crural defect.
After initial anatomic identification, an attempt is made to reduce the stomach from the hernia sac and into the abdominal cavity (Fig. 13.2). This helps to facilitate the remainder of the operation by creating additional working space in the mediastinum. A hand-over-hand technique is used to gently pull the stomach inferiorly using atraumatic graspers. However, excessive force should never be applied to the stomach during this initial maneuver. Significant adhesions can exist between the stomach and the hernia sac, and traction under these conditions can result in gastric injury and even perforation. If the stomach does not reduce easily, this step should be abandoned and the operation proceeds with dissection of the hernia sac.
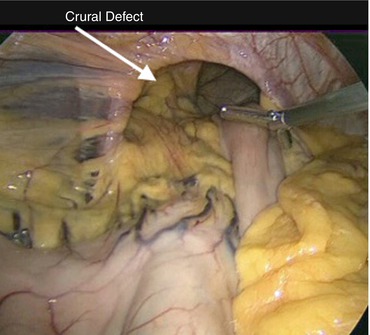

Fig. 13.2
A large PEH with a significant portion of intrathoracic stomach is seen after liver retractor placement. Gentle traction is applied to reduce as much of the stomach as possible into the abdomen prior to beginning dissection of the hernia sac
To initiate this dissection, the hepatogastric ligament is divided in order to gain access to the lesser sac and mobilize the lesser curvature of the stomach. In the case of a large type III PEH, a significant portion of the lesser curvature may lie intrathoracically. In operations involving this severe an anatomic distortion, the surgeon must be extremely careful to identify the location of the left gastric artery, right gastric artery, and even porta hepatis, prior to dividing the hepatogastric ligament, as these structures can be shifted towards the hiatus. The hepatic branch of the vagus nerve, on the other hand, can be divided without physiologic consequence.
Once the lesser sac is entered, division of the lesser omentum continues superiorly to the level of the right crus. We use an ultrasonic dissector to accomplish this, although bipolar or monopolar energy devices can also be employed. The next step is to enter the mediastinum and develop a plane on the outside of the hernia sac. The importance of this maneuver cannot be overemphasized, and the relative ease or difficulty of the remainder of the operation often hinges upon it. To achieve this, the surgeon grasps the right crus with a blunt grasper and then incises the peritoneal layer at its medial aspect (Fig. 13.3). The hernia sac is an extension of this peritoneal membrane and therefore if it is divided at the medial edge of the right crus, the mediastinum can be entered external to the sac. Once this entry is made, blunt dissection is used to sweep the sac and its contents medially and inferiorly, separating them from the rest of the mediastinal structures. The assistant forcibly retracts the hernia sac inferiorly in order to continuously reduce the hernia contents as the dissection proceeds. It should be noted that neither the surgeon nor assistant should grasp the esophagus directly, as it can be injured easily. During this portion of the procedure, the use of cautery should also be limited so as to not inadvertently cause a tear in the hernia sac or thermal injury to the esophagus or vagus nerves.
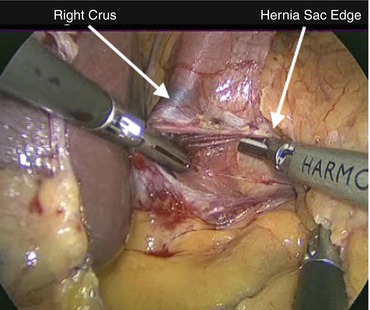

Fig. 13.3
Dissection of the hernia sac begins at the medial border of the right crus. The hernia sac and sac contents are swept to the right of the laparoscopic image, and the right crus is swept to the left in order to enter the mediastinum on the outside of the sac. The assistant provides retraction inferiorly on the hernia sac
If the correct plane has been entered, the hernia sac should separate relatively easily, revealing the right-sided mediastinal pleura laterally, pericardium anteriorly, and vertebrae and aorta posteriorly. The anterior and posterior vagus nerves should be identified as well and kept alongside the esophagus. As this mediastinal working space is enlarged, the edge of the hernia sac is sequentially divided at its junction with the crura. This is done in a clockwise direction, starting at the point of mediastinal entry and proceeding towards the left crus. Blunt dissection of the hernia sac then proceeds to the patient’s left, and the left pleura is exposed. During this step in the operation, tears in the pleura on either side can occur. This usually does not result in adverse physiologic consequences, but the anesthesiologist should be immediately informed. In the case of capnothorax that results in hypotension or increased airway pressures, a reduction in insufflation pressure, or complete deinsufflation of the abdomen, will almost always correct these abnormalities. Insertion of a chest tube is rarely, if ever, required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


