Fig. 6.1
Approximate range of odds ratios for risk factors for symptomatic peripheral arterial disease (Reproduced, with permission, from Elsevier, Norgren et al. [3])
Diabetes Mellitus (DM)
DM increases the risk of PAD three- to fourfold, doubles the risk of IC, and increases the risk of amputation several fold compared to nondiabetics [10]. The pathophysiology of diabetes includes abnormalities in the endothelium, vascular smooth muscle cells, and platelet as well as metabolic abnormalities such as hyperglycemia, increased free fatty acids, and insulin resistance [11]. Increased hemoglobin A1c (HbA1c) levels reflect chronic hyperglycemia and poorly controlled diabetes. Every 1 % increase in HbA1c level increases the risk of PAD by 26 % [3, 12].
Hypertension
Elevated blood pressure increases the risk of PAD twofold to threefold [13]. Approximately 2–5 % of patients presenting with hypertension have intermittent claudication and the prevalence increases with age. Conversely, an estimated 35–55 % of patients with PAD require treatment for hypertension [14]. Although the exact mechanism remains undefined, recent studies suggest that hypertension facilitates the development and progression of atherosclerosis by causing oxidative stress or injury to the endothelium which leads to inflammation of the arterial wall [15].
Dyslipidemia
Independent lipid risk factors for PAD include elevated levels of total cholesterol, LDL cholesterol, triglycerides, and lipoprotein(a). Extensive research suggests that the deposition, modification, and cellular uptake of cholesterol play an important role in the pathophysiology of atherosclerosis. Cholesterol injures the vascular endothelium which leads to inflammation and vessel remodeling. Vascular endothelium actively regulates vascular tone, lipid breakdown, thrombogenesis, inflammation, and vessel growth, all of which contribute to the development of atherosclerosis [16]. Elevated levels of high-density lipoprotein (HDL) cholesterol and apolipoprotein (a-1) appear to be lipid factors which protect against the development of PAD [3]. Several studies have shown that lowering cholesterol improves endothelial function, which may at least in part explain the early and substantial reduction in major cardiovascular events associated with lowering cholesterol.
Inflammatory Markers
Arterial inflammation appears to be present well before atherosclerosis is grossly visible. C-reactive protein (CRP) has been identified as a stable, easily detected marker for inflammation. Recent studies showed that higher CRP levels were positively associated with PAD, independent of other confounding risk factors including smoking, waist circumference, body mass index, blood pressure, glycosylated hemoglobin, and serum total cholesterol [17].
Hyperhomocysteinemia
An elevation in plasma homocysteine, an amino acid by-product of the demethylation of methionine, is an independent risk factor for PAD. Hyperhomocysteinemia is present in about 30 % of young patients with PAD compared to only 1 % in the general population [3].
Chronic Kidney Disease (CKD)
Patients with CKD defined by an estimated glomerular filtration rate (eGFR) between 15 and 59 ml/min per 1.73 m2 are at increased risk for developing PAD. After adjustment for cardiovascular disease risk factors, individuals with CKD had a 1.5-fold higher risk for developing PAD compared to those with normal kidney function [18].
Race
According to the NHANES (National Health and Nutrition Examination Survey) [2] and GENOA (Genetic Epidemiology Network of Arteriopathy) [19] studies, PAD is twice as common among the black population even when other risk factors such as elevated blood pressure, diabetes, and obesity are considered.,
Age and Gender
The risk of PAD increases in patients 50 years or older. Although PAD affects slightly more men than women, gender differences decrease in advanced age groups.
Diagnosis
The diagnosis of PAD usually relies on a problem-oriented history and a detailed physical examination of the vascular system. Objective, noninvasive vascular testing can then verify the clinical findings. One-third of patients with PAD present with intermittent claudication which consists of lower extremity aches, cramps, numbness, or fatigue that is induced by exercise and relieved by a short period of rest (usually less than 10 min). Although claudication symptoms usually affect the calf muscles, they may also occur in the thighs and buttocks depending on the location of the occlusive disease. Upper extremity muscle fatigue with exertion can occur in the setting of subclavian or axillary artery occlusive disease. In general, the claudication symptoms localize to one level distal to the occlusive lesion. Hence, calf claudication usually results from superficial femoral artery occlusion, while buttock and thigh claudication suggest more proximal disease of the aorta or iliac arteries.
Differentiating the symptoms of claudication from other causes of limb pain requires a thorough history including pain location and sites of radiation, relieving and aggravating factors, symptom duration, and reproducibility. A past medical history focusing on smoking and other risk factors for atherosclerosis should be obtained in addition to the social history to determine the functional and quality-of-life impact of claudication. Note that some patients with PAD do not complain of typical claudication because their severe medical comorbidities prevent them from walking enough to produce symptoms. Other uncommon vascular pathologies (e.g., popliteal artery entrapment) may also present with claudication in young patients who do not have atherosclerotic risk factors. Table 6.1 shows the differential diagnosis for intermittent claudication.
Table 6.1
Differential diagnosis of intermittent claudication
Condition | Location | Prevalence | Characteristic | Effect of exercise | Effect of rest | Effect of position | Other characteristics |
|---|---|---|---|---|---|---|---|
Calf IC | Calf muscles | 3–5 % of adult population | Cramping, aching discomfort | Reproducible onset | Quickly relieved | None | May have atypical limb symptoms on exercise |
Thigh and buttock IC | Buttock, hip, thigh | Rare | Cramping, aching discomfort | Reproducible onset | Quickly relieved | None | Impotence |
May have normal pedal pulses with isolated aortoiliac disease | |||||||
Foot IC | Foot arch | Rare | Severe pain on exercise | Reproducible onset | Quickly relieved | None | Also may present as numbness |
Chronic compartment syndrome | Calf muscles | Rare | Tight, bursting pain | After significant exercise (e.g., jogging) | Subsides very slowly | Relief with elevation | Typically affects heavily muscled athletes |
Venous claudication | Entire leg, worse in calf | Rare | Tight, bursting pain | After walking | Subsides slowly | Relief speeded by elevation | History of iliofemoral deep venous thrombosis, signs of venous congestion, edema |
Nerve root compression | Radiates down leg | Common | Sharp lancinating pain | Induced by sitting, standing, or walking | Often present at rest | Improved by change in position | History of back problems |
Worse with sitting | |||||||
Relief when supine or sitting | |||||||
Symptomatic Baker’s cyst | Behind knee, down calf | Rare | Swelling, tenderness | With exercise | Present at rest | None | Not intermittent |
Hip arthritis | Lateral hip, thigh | Common | Aching discomfort | After variable degrees of exercise | Not quickly relieved | Improved when not weight bearing | Symptoms variable |
History of degenerative arthritis | |||||||
Spinal stenosis | Often bilateral buttocks, posterior leg | Common | Pain and weakness | May mimic IC | Variable relief, but can take a long time to recover | Relief by lumbar spine flexion | Worse with standing and spine extension |
Foot/ankle arthritis | Ankle, foot arch | Common | Aching pain | After variable degrees of exercise | Not quickly relieved | May be relieved by not bearing weight | Variable; may relate to activity level and present at rest |
The physical examination should include a comprehensive vascular assessment because patients often have arterial disease affecting more than one area. Essential components of the exam include blood pressure measurements in both arms, a cardiac assessment, and a detailed abdominal examination to evaluate for an abdominal aortic aneurysm. Both lower extremities should be examined and compared to each other. Some less specific findings of PAD include pallor with elevation/dependent rubor (associated with critical limb ischemia), decreased hair growth, coolness in the most distal aspect of the extremity, dystrophic nails, and occasionally muscle atrophy. Bruits suggesting turbulent arterial flow may be heard over the carotid arteries, abdominal aorta or its branches, and femoral arteries. A detailed examination of the right and left radial, ulnar, brachial, carotid, femoral, popliteal, dorsalis pedis, and posterior tibial pulses should be done and compared side to side. Vessel wall calcification may be palpable (i.e., firm vessel with limited pulsatility) in patients with long-standing renal failure and diabetes, while patients with arteritis may have vessels that are tender to palpation. Pulse strength should be graded as absent, weak, normal, or aneurysmal, and the capillary refill time noted. In patients who present with severe PAD, Buerger’s elevation test usually demonstrates pallor on elevation and dependent rubor (“sunset sign”). A key component of the examination is determining the presence or absence of a femoral pulse. A non-palpable femoral pulse usually indicates aortoiliac disease or common femoral artery occlusion, and this finding often helps to determine further imaging choices and treatment options. A normal femoral pulse with absent popliteal and pedal pulses typically indicates superficial femoral arterial disease, while palpable femoral and popliteal pulses with absent pedal pulses often indicate infrapopliteal occlusive disease (the latter frequently occurring in diabetic patients). A handheld Doppler probe should be used for documentation if pulses are not palpable.
Work-Up
Although the clinical history often suggests the diagnosis of PAD, physical exam and pulse evaluation may not be reliable enough to make the diagnosis with confidence. Criqui et al. showed that only 18 % of patients with abnormal posterior tibial pulses had additional objective evidence of PAD [20]. Noninvasive screening tests and imaging studies help confirm the diagnosis by providing accurate and reproducible findings which support the diagnosis of PAD.
The ankle-brachial index (ABI) is a simple and accurate test for detecting PAD that can be performed in the office or at the bedside. Measuring the ABI involves using a handheld Doppler probe and manual blood pressure cuff to record the systolic blood pressure at the ankle and in the arm. The blood pressure cuff should be placed just above the ankle while locating the posterior tibial artery or dorsalis pedis artery with the Doppler probe. While maintaining the Doppler signal, the blood pressure cuff is inflated until the signal is obliterated. The cuff is then slowly deflated and the pressure at which the Doppler signal returns is the systolic ankle pressure. The same steps are repeated for the remaining pedal artery. The brachial pressure is obtained in the same manner with a blood pressure cuff on the upper arm and a Doppler probe on the radial or ulnar pulse. To calculate the ABI, the highest systolic pressure measured at the ankle is divided by the higher of the two systolic brachial pressures (Fig. 6.2). Using the higher brachial pressure for both lower extremities ensures that the ABI will not be underestimated in patients with upper extremity blood pressure discrepancy due to subclavian artery stenosis.
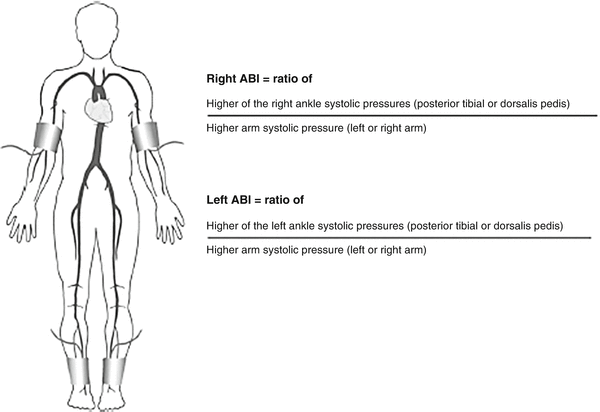

Fig. 6.2
Calculation of the ankle-brachial index (ABI)
The ABI correlates with the presence and severity of occlusive disease with a normal ABI ranging from 0.90 to 1.3; an ABI of 0.70–0.90 indicates mild disease; 0.40–0.70 moderate disease; and an ABI less than 0.40 indicates severe occlusive disease [21]. An ABI greater than 1.3 should raise suspicion that the arterial wall is stiffened by medial calcinosis, as often occurs in diabetics. In some patients with moderate wall calcification, a normal ABI may not reflect the true perfusion to the lower extremities. A falsely elevated ABI should be suspected in patients with a strong clinical suspicion for PAD or typical claudication symptoms who have absent palpable pedal pulses despite a normal or near normal ABI. This finding should prompt further testing with waveform analyses or imaging studies.
Most patients with intermittent claudication have an ABI between 0.5 and 0.9, while those with ischemic rest pain have an ABI less than 0.4, and patients with impending gangrene have an ABI less than 0.3. Common mistakes to avoid when taking an ABI include not using the higher of the two arm pressures as the denominator, failure to have the patient supine for at least 5 min to allow stabilization of blood pressure and failure to choose an appropriately sized cuff (the bladder length of the cuff should be 80 %, and the width should be 40 % of the circumference of the extremity).
The noninvasive vascular lab can perform several tests that evaluate PAD in more detail and may help confirm the diagnosis in patients with atypical clinical presentations. Segmental limb pressures can detect and localize arterial occlusive lesions by using cuffs placed at the arm, at the upper thigh, above the knee, below the knee, and at the ankle. A pressure drop of 20 mmHg or more at any level in comparison to the proximal or contralateral level indicates significant arterial disease. Occasionally, well-developed collateral vessels can compensate for occlusive disease and minimize the pressure disparity. Inaccurate readings may also result from using an undersized cuff on the thigh which falsely elevates the pressure, masking iliac artery stenosis.
Pulse volume recording (PVR) offers another alternative to detect and localize PAD. Cuffs placed on the lower extremity at multiple levels are inflated to 65 mmHg and the pulse waveforms are recorded. A normal pulse waveform consists of a rapid upslope, a sharp systolic peak, a dicrotic notch, and a gentle bow toward the baseline. In the presence of PAD, the waveform becomes dampened and the degree of dampening usually correlates with the severity of the stenosis. Compared to segmental pressure measurements, PVR is less affected by arterial calcification and combining PVR and segmental pressure measurements increases the overall accuracy for detecting PAD [22].
Although exercise testing is rarely required to diagnose PAD, it can distinguish arterial claudication from pseudoclaudication. As a physiologic study, exercise testing can also determine the extent to which underlying conditions such cardiopulmonary, orthopedic, or muscular disease contribute to the patient’s symptoms. The patient usually rests for 20 min before measuring the resting ABI. The patient then walks on a treadmill at a fixed speed (2 mph) and inclination (10–12°) for 5 min or until claudication symptoms develop. Repeated toe raises can substitute for walking when a treadmill is not available. The patient then reclines and the ankle and arm pressures are measured immediately and repeated every 2 min for 10 min or until the pressure returns to resting levels. In the supine position, a decrease in ABI of 15–20 % is diagnostic for PAD. Patients with severe aortic stenosis, uncontrolled hypertension, severe congestive heart failure, unstable angina, or chronic obstructive pulmonary disease should not undergo exercise testing.
Digital pressure measurements can be used to detect PAD in patients who have noncompressible vessels or falsely elevated ABI’s (typically due to diabetes or ESRD). The toe-brachial index (TBI) helps to provide a frame of reference as the normal toe pressure is 20–40 mmHg lower than the ankle pressure. A normal TBI is greater than 0.7, a TBI of 0.64–0.7 is borderline, and a TBI less than 0.64 is clearly abnormal [23]. A toe pressure less than 30 mmHg is associated with ischemic symptoms.
In most patients, the combination of clinical history, physical exam, and noninvasive tests can establish the diagnosis of PAD and determine the level of occlusive disease. Imaging studies are not required to confirm the diagnosis of PAD, nor should they be used as first line screening tests as they do not alter the natural history of the disease or the initial management strategy. The primary role of imaging studies involves treatment planning for patients who are being considered for revascularization. Chapter 8 has a more detailed description of the most common imaging modalities including duplex ultrasound, computed tomography angiography (CTA), magnetic resonance angiography (MRA), and catheter-directed angiography.
Treatment Indications and Options
The management of PAD poses a challenge because it represents a systemic disease with a wide range of clinical severity. Developing an individualized treatment plan for patients with claudication requires knowledge of the natural history of PAD, as well as a thorough evaluation of each patient’s unique comorbidities, including extent of systemic atherosclerosis, functional capacity, and cardiovascular risk factors (Fig. 6.3). Most patients with intermittent claudication have a slow decrease in walking distances and rarely progress to limb-threatening ischemia (rest pain, ischemic ulcer, or gangrene) especially when risk factors are controlled. Only 25 % of patients with claudication demonstrate any clinical deterioration of the limb perfusion, and the risk of major amputation in a claudicant ranges from 1 to 7 % over 5 years [24–26]. Decisions on the timing and type of intervention depend on the extent of anatomic involvement, the available revascularization options, and the expected benefits of intervention balanced against early and late risk. The patient with claudication should clearly understand that they do not have limb-threatening ischemia and that intervention is not required for immediate limb salvage or prevention of amputation in the future. Treatment strategies for patients with claudication should focus on controlling cardiovascular risk factors, increasing walking distance, and improving the quality of life. Achieving these goals requires an individualized treatment plan based on the extent of disability and functional needs of each patient. Success in managing patients with claudication involves education regarding what to expect from each proposed treatment and encouraging active participation in the decision-making process.
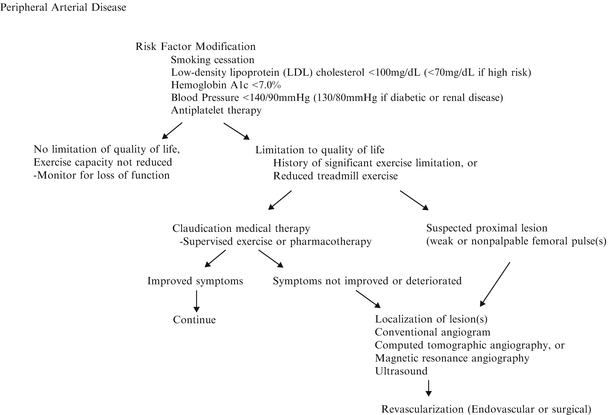

Fig. 6.3
Overall treatment strategy for peripheral arterial disease. BP blood pressure, HbA1c hemoglobin A1c, LDL low-density lipoprotein, MRA magnetic resonance angiography, CTA computed tomographic angiography
Since PAD (ABI < 0.9) is a manifestation of systemic atherosclerosis, it is not surprising that it has a strong association with CAD and stroke. In a study on the fate of 2,777 male claudicants, the mortality rate was 42 and 65 % at 5 and 10 years, respectively, with myocardial infarction accounting for two-thirds of the 1,363 deaths [26]. A decrease in ABI correlated with an increase in the incidence of cardiac and cerebrovascular disease in a population study of 13,678 patients over a 13-year period [26]. In another large study on patients older than 70 or age 50–69 with diabetes or smoking history, 16 % of patients with an ABI less than 0.9 had symptomatic CAD or cerebrovascular disease [27]. The ACC/AHA guidelines emphasize the fact that the most significant risk facing patients with claudication is cardiac death, not limb loss. The risk of cardiac death for claudicants is 3–5 % per year compared to a 1 % risk of amputation [28].
Medical Management
Risk Factor Modification:
All patients with PAD require cardiovascular risk factor modification irrespective of the treatment plan for their claudication symptoms.
Smoking
Smoking cessation is the cornerstone of any treatment plan for claudication. Active smokers should be educated so that they understand that none of their other medications surpasses the importance of smoking cessation. Smoking cessation decreases the risk of death, myocardial infarction, amputation, and lower extremity intervention. Patients who quit smoking significantly improve their exercise time compared to those who continue to smoke [4, 29]. The issue of smoking cessation should be discussed at each clinical encounter with patients who smoke. Unfortunately, physician advice and frequent follow-up achieved a quit smoking rate of only 5 %. Not surprisingly an intensive smoking cessation program with individual counseling and pharmacologic therapy resulted in better quit rates compared to verbal advice alone (21 % vs. 7 %) [30, 31]. The addition of medications such as bupropion and varenicline or nicotine replacement can provide valuable assistance to patients attempting smoking cessation. Varenicline acts as a partial agonist of α4β2 nicotine acetylcholine receptor, which minimizes the effect of withdrawal by releasing dopamine to reduce craving. In randomized clinical trials, varenicline had superior quit rates compared to nicotine replacement and bupropion [32]. At 9 weeks, varenicline had a quit rate of 44 % versus 16 % for nicotine replacement, 30 % with bupropion, and 35 % with both bupropion and nicotine replacement [33].
Hyperlipidemia
The current guidelines by ACC/AHA recommend an LDL cholesterol level of less than 100 mg/dl in patients with PAD and less than 70 mg/dl in those with evidence of generalized atherosclerosis. Lipid-lowering agents, especially statins (3-hydroxy-3-methyglutaryl coenzyme A (HMG-CoA)), have been increasingly used as they have been shown to decrease cardiac-related events. The beneficial effects of statins appear to extend beyond their lipid-lowering properties. These pleiotropic properties include the ability to stabilize atherosclerotic plaque while decreasing oxidative stress, vascular inflammation, and platelet aggregability [34].
The Heart Protection Study (HPS) [35] randomized 20,500 patients (6,748 with PAD) to simvastatin 40 mg, antioxidant vitamins, a combination of the two, or placebo. Patients who received 40 mg of simvastatin had a significant reduction in overall mortality (12 %), vascular mortality (17 %), coronary events (24 %), all strokes (27 %), and noncoronary revascularization (16 %). Results were similar in the PAD subgroup. In a meta-analysis of statin therapy [36], a 39 mg/dl reduction in LDL was associated with a 20 % decrease in major cardiovascular event risk independent of the initial lipid levels, including those with normal lipid levels.
Patients with PAD may also have abnormal HDL and triglyceride metabolism. Increasing low HDL levels (<40 mg/dl) using fibrates or niacin benefits patients with coronary artery disease by reducing the risk of nonfatal MI, cardiovascular death, and progression of coronary atherosclerosis [37–39].
Dietary modification should be the initial intervention to control abnormal lipid levels. In addition, patients who are overweight (BMI 25–30) or obese (BMI >30) should be counseled for weight reduction and referred for weight reduction programs if available.
Diabetes Mellitus
Although the microvascular complications of diabetes (retinopathy, nephropathy) increase with uncontrolled blood glucose levels, strict glucose control does not seem to decrease the rate of macrovascular complications, particularly PAD. Nevertheless, the current American Diabetes Association guidelines recommend hemoglobin A1c <7 % as a treatment goal for all patients with DM [39, 40].
Hypertension
The current guidelines recommend a target blood pressure of less than 140/90 mmHg in high risk groups such as those with PAD and less than 130/80 mmHg in patients who also have diabetes or renal insufficiency [41, 42]. The benefit of blood pressure control on decreasing the risk of cardiovascular events does not seem to be linked to the specific medication used. Often more than one medication is needed to achieve this goal. Thiazide diuretics are first line agents, and ACE inhibitors or angiotensin receptor blockers are recommended in patients with diabetic renal disease or congestive heart failure. A subgroup analysis of the 4,046 patients with PAD in the Heart Outcomes Prevention Evaluation (HOPE) study showed a 22 % decrease in the risk of stroke, MI, and vascular mortality in patients randomized to receive an ACE inhibitor (ramipril) [43]. This finding which was independent of the absolute reduction in blood pressure provides support for the use of ACE inhibitors as a treatment for hypertension in patients with PAD. Beta-adrenergic blocking medications can also be used for patients with PAD, especially those with coronary artery disease. Randomized trials have refuted the previously held belief that these medications could exacerbate claudication symptoms.
Homocysteine
Although an elevated homocysteine level is associated with PAD, treatment of patients with high doses of folic acid is not recommended as it has failed to show any benefit [3]. Checking homocysteine levels and giving supplemental folic acid or B vitamins may be appropriate in patients with a family history of multiple thrombotic events or premature cardiovascular events in the absence of conventional atherosclerotic risk factors.
Antiplatelet Drug Therapy
All patients with claudication should receive antiplatelet therapy because it reduces the risk of MI, ischemic stroke, and vascular mortality. The Antithrombotic Trialists’ Collaboration which included 102,459 patients with cardiovascular disease showed that the risk of cardiovascular events in patients treated with aspirin-(acetylsalicylic acid, ASA) was 9.5 % versus 11.9 % in the control group. Patients with claudication had an 18–23 % decrease in cardiovascular events in a subgroup analysis [44]. Low dose (75–150 mg) ASA has proved to be as effective as higher dose aspirin, without the increased risk of gastrointestinal bleeding.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


