13 Pacing for Sinus Node Disease
Sinus node disease (SND) is the most common indication for a cardiac pacing system.1 SND increases exponentially with age, affecting 1 of every 600 cardiac patients older than 65. SND is characterized by electrophysiologic abnormalities of both the sinus node and the atria. These electrophysiologic abnormalities include disturbances of impulse generation and exit from the sinus node to atrial tissue, impaired impulse transmission within the atria and specialized cardiac conduction system, failure of subsidiary pacemaker activity, and paroxysmal or chronic atrial tachycardias, including atrial fibrillation.2,3 The electrocardiographic manifestations of SND include (1) sinus bradycardia, (2) sinus pauses or sinus arrest, (3) sinoatrial exit block, (4) atrial tachycardia, (5) atrial fibrillation (initially paroxysmal), and (6) sinus node chronotropic incompetence.2,3 Bradyarrhythmias alternating with paroxysmal atrial flutter or fibrillation are common in SND. The natural history of SND includes recurrent syncope, heart failure, stroke, and atrial fibrillation.
 Pathophysiology and Diagnosis
Pathophysiology and Diagnosis
Cellular Electrophysiology
The sinoatrial node is a heterogeneous tissue with multiple cell types and a complex structure.3,4 The action potential governing pacemaking usually arises in the center of the sinoatrial node and propagates into the surrounding muscle of the right atrium. A variety of ionic currents contribute to normal sinus node automaticity (Fig. 13-1). These include If, a hyperpolarization-activated inward current, and the L– and T– type calcium currents (ICaL, ICaT). At present, debate remains over which of these currents is dominantly responsible for sinus node automaticity.5 There is limited or no measurable sodium current (INa) in the center of the node, but it is measurable in the periphery.3,4 INa may be responsible for activating peripheral cells. Outward currents that govern sinus node automaticity include the sodium-potassium (Na+,K+) pump, the delayed rectifier current (IKr), and the potassium channel activated by the muscarinic receptor, IKACh. The sodium-calcium exchanger and sarcoplasmic reticulum (SR)–mediated calcium ion (Ca2+) release also influence sinus node automaticity. Abnormalities of one or more of these ionic currents may contribute to sinus node dysfunction. During aging, connexin 43 (Cx43) disappears from the sinus node, which may contribute to abnormalities of conduction within the sinus node.3,6
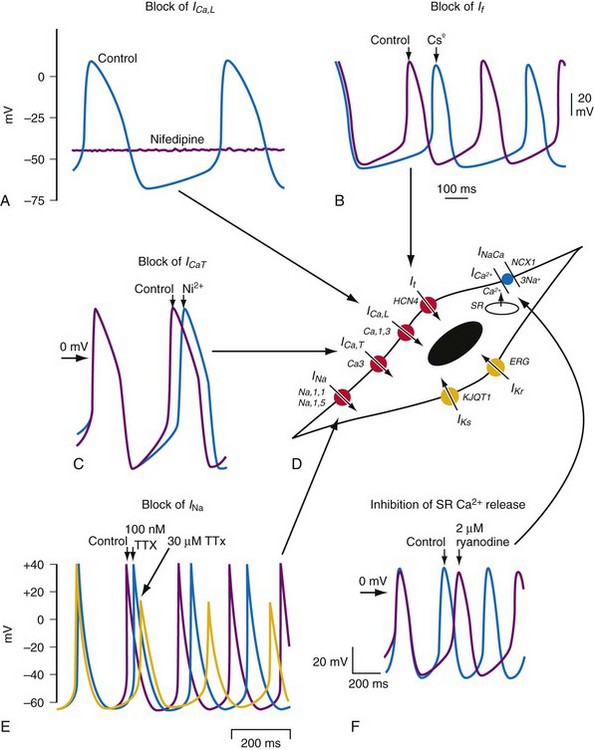
Figure 13-1 Pacemaking mechanisms.
(From Dobrzynski H, Boyett MR, Anderson RH: New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation 115:1921-1932, 2007.)
The molecular architecture of the human sinus node has recently been described.4 A complex and heterogeneous expression of ion channels is present within the sinus node compared to a paranodal region within the terminal crest and right atrial (RA) tissue. There is a lower expression of Nav1.5, Kv4.3, Kv1.5, HERG, Kir2.1, Kir6.2, the cardiac ryanodine receptor, the SR calcium pump, connexin 40 (Cx40), and Cx43 messenger ribonucleic acid (mRNA), but a higher expression of Cav1.3, Cav3.1, hyperpolarization-activated cyclic nucleotide–gated channel genes (HCN1, HCN4) mRNA in the sinus node compared to RA tissue (Figs. 13-2 and 13-3). Similar differences in expression of the corresponding ion channel proteins are observed in the sinus node compared to RA tissue. The expression pattern of many ion channels in the paranodal area is intermediate between that of the sinus node and right atrium, although greater expression of Kv4.2, Kir6.1, minK-related peptides, and other potassium channels (TASK1, SK2) is observed in the paranodal area than in the sinus node or atrial tissue. Estimating conductance of the key ionic currents based on a percentage of the mRNA levels expressed in the sinus node, then incorporating these in a mathematical model of human atrial action potential, successfully produced a model of pacemaking.4
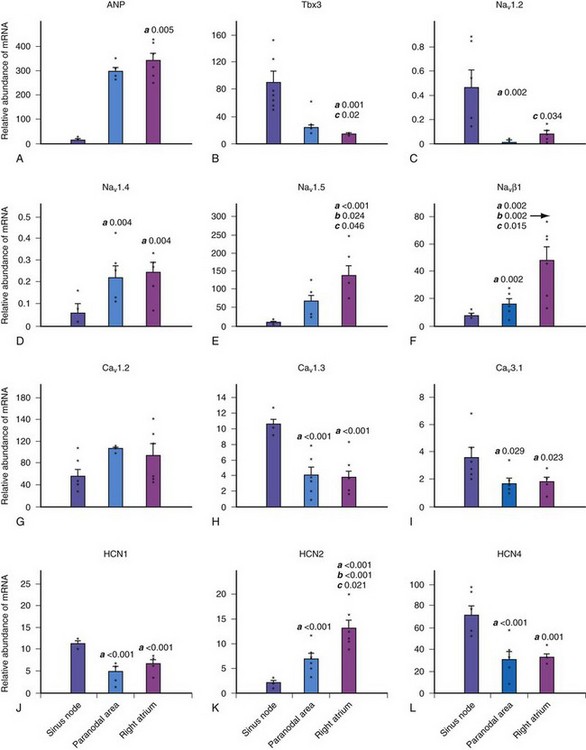
Figure 13-2 Relative abundance of mRNA in sinus node.
(From Chandler NJ, Greener ID, Tellez JO, et al: Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation 119:1562-1575, 2009.)
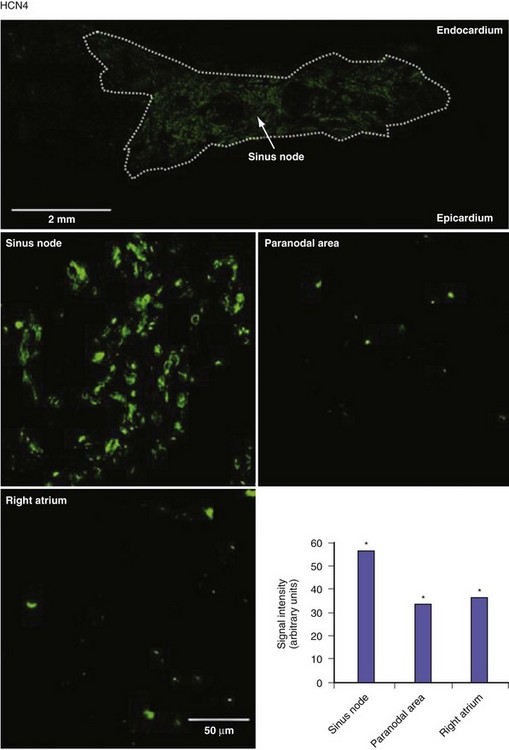
Figure 13-3 Expression of HCN4 protein in sinus node, paranodal area, and right atrium.
(From Chandler NJ, Greener ID, Tellez JO, et al: Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation 119:1562-1575, 2009.)
Abnormalities of one or more ionic channels, transporters, or receptors that are inherited or that develop under pathophysiologic states, including aging or atrial fibrillation (AF), likely contribute to the substrate for SND.3 Knockout of various ion channels (Nav1.5, sodium channel β2-subunit, Cav1.3, Cav3.1, HCN2, HCN4, ankyrin-B) and gap-junction subunits (Cx40) in transgenic mice result in a SND phenotype characterized by bradycardia, sinus dysrhythmia and sinus node exit block.3–7 Mutations of the HCN4 gene have been associated with reduced membrane expression and decreased If currents in conjunction with abnormalities of sinus node dysfunction.8–10 In an experimental model of heart failure, decreases in the intrinsic sinus rate have been observed in association with significant decreases in If.11 Mutations of the alpha subunit of the sodium channel that causes one form of the long QT syndrome have also been associated with SND.12–14 Such mutations may lead to the presence of a persistent inward current and a negative shift in voltage-dependence of inactivation that either separately or in combination may cause a reduction in the sinus rate. Congenital SND that is characterized by bradycardia progressing to atrial inexcitability has also been associated with mutations of the α-subunit of the sodium channel that cause loss of function or significant impairments in channel gating (inactivation), resulting in reduced myocardial excitability.15 SND has also been linked to mutations in ankyrin-B, a protein important in the regulation of ion channel function.16,17
Knockout of HERG1 B, the gene that encodes IKr, in mice has also been associated with episodic sinus bradycardia.18 Mutations of the KCNQ1 gene that encodes the KvLQT1 potassium channel are also associated with SND.19 Thus, decrease or loss of IKr or IKs in the long QT syndrome may contribute to SND in that setting. Maternal antibodies may contribute to sinus node dysfunction in the setting of congenital complete heart block by inhibition of ICaL and ICaT.20 Knockout of ICaL in transgenic mice has been associated with SND.3,21,22
Some experimental models of ventricular hypertrophy and heart failure have been reported in association with sinus node dysfunction and AF.23–27 Because SND is age dependent, changes in sinus node function may be secondary to changes in ion channels or gap junctions. Loss of INa in the periphery of the sinus node and decreased expression of Cx43 have been reported in animal models of aging and may explain abnormalities of sinus node function.3 Thus, the causes of SND are likely multifactorial.
Clinical Electrophysiology
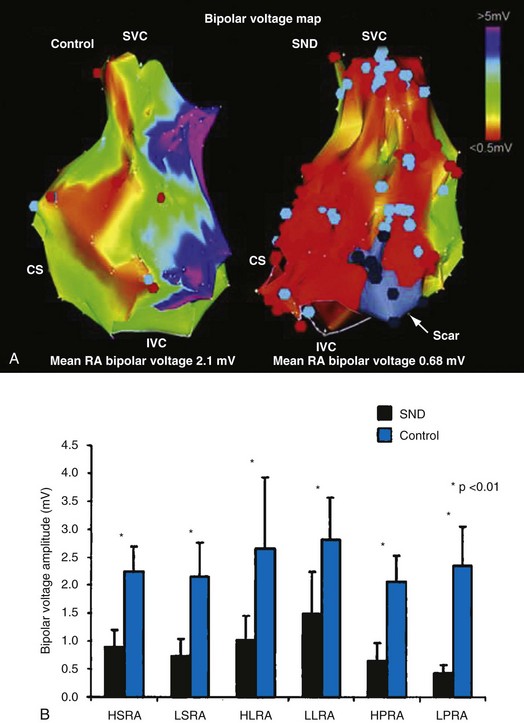
In normal hearts, the sinus node complex displays a dynamic range of activation sites along the posterolateral right atrium.28 There are multiple origins of sinus activation and exit sites to the atria, providing evidence of multicentricity of the sinus node complex. Electroanatomic mapping has demonstrated that preferential pathways of conduction exist between the sinus node and the exit of sinus activity to the atria. In SND, the sinus node complex more often is unicentric and localized to the low crista terminalis.29,30 Electroanatomic mapping in patients with SND has also demonstrated significant increases in atrial refractory periods at all RA sites, increased atrial conduction times along the lateral right atrium and coronary sinus, and greater number and duration of double potentials along the crista terminalis29 (Fig. 13-4).29
Significant regional conduction slowing with double potentials and fractionation associated with areas of low voltage and electrical silence has also been observed in the right atrium. The slow conduction may be caused by increased fibrosis in the atria, although other determinants of conduction velocity (e.g., INa or connexin expression) have not been studied in patients with SND. Areas of low voltage/scarring in the right atrium can be widespread, progressive, and severe. Similar changes are seen in conditions of chronic atrial stretch and increasing age.30 The mechanism of these changes in patients with SND is unclear. These results indicate not only that there is disease of the sinus node, but that the atrial electrophysiologic abnormalities also extend to the RA myocardium.
Patients with congestive heart failure (CHF) manifest significant sinus node remodeling, characterized by anatomic and structural changes along the crista terminalis, and a reduction in functional sinus node reserve.31,32 Compared to age-matched controls, patients with CHF have greater prolongation of the intrinsic sinus cycle length, more prolonged sinus node recovery time, caudal origin of sinus activity, prolongation of sinoatrial conduction time, greater number and duration of fractionated electrograms or double potentials along the crista terminalis, loss of voltage amplitude along the crista, and abnormal and circuitous propagation of the sinus impulse. In addition, atrial refractoriness and regional conduction times are prolonged, and these changes may contribute to the propensity to AF in this setting.32
Experimental and clinical data suggest that both AF and atrial flutter cause adverse remodeling of the sinus node.33–39 Sinus node activation in patients with persistent atrial flutter is characterized by more caudal activation, slower conduction time along preferential pathways, and modest shifts within the functional pacemaker complex. Prolonged sinus pauses after paroxysms of AF may result from depression of sinus node function that is eliminated by curative ablation of AF.38 Sinus node recovery times are prolonged immediately following successful cardioversion and shorten over time.39 In an experimental model of atrial tachyarrhythmias, downregulation of HCN2, HCN3, and minK subunit expression along with the corresponding currents If and IKs has been reported.40 These changes were associated with abnormalities of sinus node function, whereas the L– and T-type calcium units and Cx43 were unaffected. These data suggest that changes in If and IKs contribute to the abnormalities of sinus node function associated with AF.
The long-term loss of atrioventricular (AV) synchrony induced by VVI pacing is also associated with atrial electrical remodeling, characterized by nonuniform prolongation of atrial refractoriness and prolonged atrial conduction and sinus node recovery times.33 These electrophysiologic changes are reversible after restoration of AV synchrony with DDD pacing. Together, the changes in atrial and sinus node electrophysiology may contribute to the higher incidence of AF observed in patients treated with VVI pacing versus AV sequential pacing. The cellular mechanisms resulting in the association between sinus node dysfunction and atrial tachyarrhythmias (tachycardia) at present remain unknown.
Clinical Presentation
The most common symptoms for which patients with SND seek medical attention include presyncope, syncope, palpitations, decreased exercise tolerance, and fatigue2,3 (Table 13-1). Symptoms are usually intermittent and may be of variable duration. Many patients with electrocardiographic evidence of sinus node disease may be asymptomatic. Symptoms secondary to systemic thromboembolism may also be observed. Syncope may be secondary to profound sinus bradycardia, asystole, or atrial tachycardias. Syncope may occur without warning or may be heralded by dizziness or palpitations. The physical examination is frequently unremarkable, although sinus bradycardia or AF should raise suspicion of SND. However, sinus bradycardia is frequently observed in normal, healthy individuals of all age ranges. Clinical correlation with symptoms is important.
TABLE 13-1 Symptoms of Sinus Node Disease
| Type | Symptoms |
|---|---|
| Major | |
| Less specific |
Diagnosis
Table 13-2 summarizes the diagnostic tools presently available for SND. Because of the intermittent nature of this syndrome, the diagnosis is often time-consuming and frustrating. The sensitivity of both noninvasive tests and invasive electrophysiologic studies to identify SND as a potential cause of syncope are low (4%-16%).2 Implantable loop recorders have increased the likelihood of diagnosing bradycardia as the cause of syncope in patients with SND, and this approach in select patients is more cost-effective than a strategy of serial noninvasive studies followed by invasive studies.41–43
TABLE 13-2 Diagnostic Evaluation of Sinus Node Function
| Diagnostic Mode | Device/Measurement |
|---|---|
| Electrocardiogram (ECG) | |
| Exercise treadmill testing | |
| Autonomic testing | |
| Invasive electrophysiologic assessment |
Natural History
The course of SND is unpredictable; periods of symptomatic sinus node dysfunction may be separated by long periods of normal function.2 SND is believed to evolve over 10 to 15 years, commencing with an asymptomatic phase and ultimately progressing to complete failure of sinus node activity and the emergence of subsidiary pacemaker escape rhythms or the development of chronic AF. Menozzi et al.44 performed a prospective study in 35 untreated patients with SND.44 These subjects had a mean sinus rate at rest of 50 beats per minute or less (≤50 bpm) and/or intermittent sinoatrial block, as well as symptoms attributable to SND. The patients were followed for up to 4 years (mean, 17 ± 15 months). During follow-up, 57% of patients experienced at least one cardiovascular event that required treatment. Syncope occurred in 23%, symptomatic heart failure in 17%, permanent AF in 11%, and symptomatic paroxysmal atrial tachyarrhythmias in 6%. The rates of cardiovascular events were 35%, 49%, and 63% after 1, 2, and 4 years, respectively. Older age and associated left ventricular dysfunction were independent predictors of cardiovascular events. The rate of syncope was 16%, 31%, and 31% after 1, 2, and 4 years, respectively. Although a favorable outcome was observed in 43% of patients, the cohort studied was small and duration of follow-up relatively short.
In a cohort of 213 patients with symptomatic SND treated with atrial pacing, the incidence of permanent AF during follow-up was 1.4% per year.45 The risk of developing AF increased substantially in patients age 70 or older at pacemaker implantation. The risk of high-grade AV block was 1.8%/yr and was much greater in patients with complete bundle branch block or bifascicular block (35%) than in patients without such conduction disturbances (6%). Survival rates in this population were similar to those of a matched general population (97% at 1 year, 89% at 5 years, 72% at 10 years). Retrospective data also support the conclusion that survival in patients with SND treated with pacemaker therapy is similar to a general matched population.46
 Clinical Outcomes in Sinus Node Disease
Clinical Outcomes in Sinus Node Disease
Pacing and Survival
A number of retrospective studies47 and one prospective study48 have reported that atrial-based pacing compared to ventricular pacing is associated with improved survival in patients with symptomatic bradycardia secondary to SND. Andersen et al.48 randomized 225 patients (mean age 76 years) with sinus node dysfunction, normal AV conduction, and a normal QRS complex to AAI or VVI pacing. Over a mean of 5.5 years of follow-up, fewer cardiac deaths occurred in the atrial pacing group (19%) compared to the ventricular pacing group (34%; P = .0065). The annual mortality rate was 3% in the atrial versus 6.4% in the ventricular pacing group.
However, these results have not been confirmed in three larger clinical trials. The Pacemaker Selection in the Elderly (PASE) trial randomized 407 patients age 65 and older to receive a dual-chamber pacemaker programmed to either the DDDR or the VVIR mode.49 Overall mortality was similar in the two pacing groups. Of the 175 patients with SND as the primary indication for pacing in this study, overall mortality was slightly higher in the VVIR group (8.8%/yr) than the DDDR group (5.2%/yr; P = .09).
The Canadian Trial of Physiologic Pacing (CTOPP) randomized 2568 patients from a general pacemaker population, 42% with SND as an indication for pacing but without permanent AF, to receive a ventricular (1474) or atrial-based (1094) pacemaker.50 Over a mean follow-up of 3.1 years, overall mortality was similar in both groups (6.3%/yr in physiologic vs. 6.6%/yr in ventricular pacing group; P = 0.92). Since the effects of atrial pacing for prevention of AF were delayed in the CTOPP population, and similar observations were reported by Danish investigators,48 the follow-up in CTOPP was extended. Over 6.4 years, the primary composite outcome of cardiovascular death or stroke occurred at 6.1%/year in patients assigned to ventricular pacing and 5.5%/year in those assigned to physiologic pacing.51 The relative risk reduction was 8.1% with physiologic pacing (95% CI = −6.5 to 20.7; P = 0.26).
The Mode Selection Trial (MOST) investigators randomized 2010 patients with SND to rate-modulated ventricular or dual-chamber pacing.52 Over a mean follow-up of 2.76 years, annual mortality was similar in both groups: 7.1% in the physiologic versus 7.4% in the ventricular pacing group (P = 0.65) (Fig. 13-5).
A pooled analysis of five randomized trials did not show a significant reduction in mortality (hazard ratio [HR] = 0.95; 95% CI = 0.87 to 1.03) associated with atrial or dual-chamber pacing versus ventricular pacing.53 In a population-based cohort of 8777 patients with SND treated with AAI or DDD pacemakers, a slight increase in all-cause mortality (HR = 1.12; CI = 1.00 to 1.25), was seen among DDD patients compared to AAI patients.54
Pacing and Atrial Fibrillation
Paroxysmal AF, atrial flutter, and atrial tachycardia (AT) occur frequently in SND.55 AT/AF detection and data storage features are now present in most dual-chamber pacemakers, facilitating the diagnosis and treatment of AF.55–61 Some devices have special algorithms developed specifically for the prevention and management of atrial tachyarrhythmias.58–61
Detection of Atrial Fibrillation
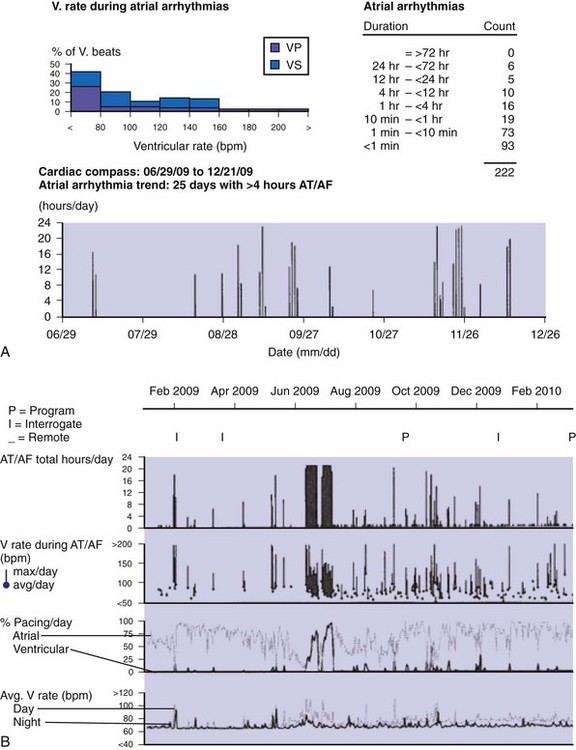
Accurate detection of atrial tachyarrhythmias, including atrial flutter and AF, by implantable devices with advanced AT management features is important for several reasons. Accurate detection ensures the appropriateness of automatic mode switching and of atrial antitachycardia pacing (ATP) for atrial flutter. Many implantable devices provide a wide range of diagnostic data, including the frequency of AT/AF, time of onset and duration of episodes, quantity of AT/AF (burden expressed as % of day or hours/day), ventricular rate control during AF, and symptomatic events56–58 (see Fig. 13-5). Such information may be valuable for managing antiarrhythmic drug therapy62–64 and when considering the need for antithrombotic therapy.65,66 Patients with AF associated with episodes of rapid ventricular rates are more likely to experience hospitalization for cardiovascular events63 (Fig. 13-6). Identification of such episodes by an implanted cardiac device can prompt changes in antiarrhythmic drug therapy.
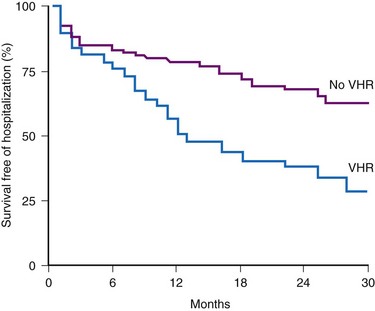
Figure 13-6 Survival free from hospitalization for cardiovascular symptoms.
(From Willems R, Morck ML, Exner DV, et al: Ventricular high-rate episodes in pacemaker diagnostics identify a high-risk subgroup of patients with tachy-brady syndrome. Heart Rhythm 1:414-421, 2004.)
With advances in remote monitoring of pacemakers and implantable defibrillators, early detection of AT may facilitate earlier medical intervention.67 Many recent clinical device trials have used device-based indices of arrhythmia recurrence, frequency, and burden as surrogates for clinical endpoints.56 Although measures of AT/AF burden in devices is quite accurate, counts of AT/AF frequency are less accurate because of variations in device-based criteria for detection, termination, and redetection, as well as intermittent atrial undersensing during AT/AF episodes.68,69
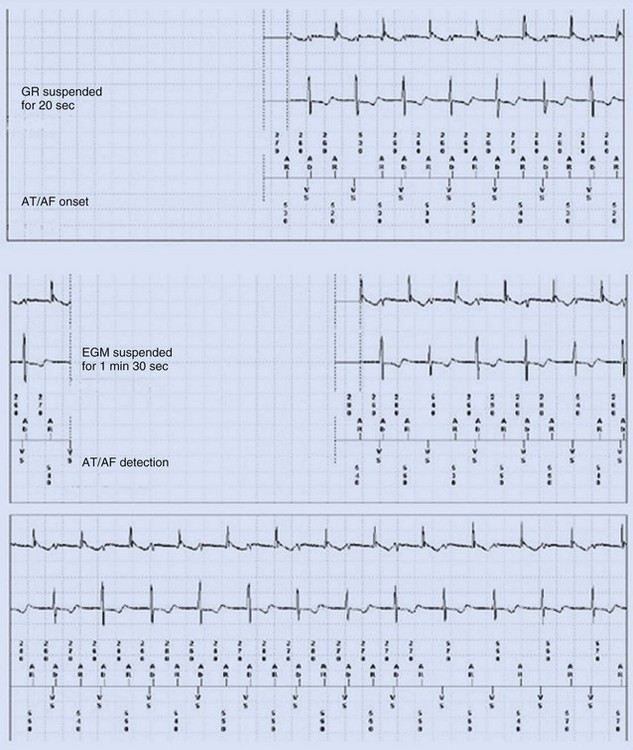
Some investigators report comparatively low values for appropriate AT/AF episode detection using early-generation pacemaker mode-switching and detection algorithms.70 Table 13-3 summarizes factors influencing AT/AF underdetection or overdetection. It is important that pacemakers be programmed to optimize detection of AT/AF, with careful attention to the atrial sensitivity and post–ventriculoatrial blanking period. Atrial undersensing from inappropriate noise reversion has been described when high atrial sensing levels are programmed.71 Atrial lead position is an important factor for appropriate AT/AF detection because some sites (e.g., near coronary sinus os, within RA appendage) are associated with a high incidence of far-field R-wave (FFRW) oversensing that may be inappropriately classified as AF. Figure 13-7 shows an example of inappropriate classification caused by FFRW oversensing. Interelectrode lead spacing (e.g., 5 mm) may also minimize FFRW sensing.
TABLE 13-3 Factors Influencing Device Detection of AT/AF
| Sensing | Factors |
|---|---|
| Undersensing | |
| Oversensing |
AT/AF, Atrial tachycardia/fibrillation; EGM, electrogram.
Attention to these issues promotes high sensitivity and specificity of AT/AF detection.72 Applying these parameters, sensitivity of AT/AF detection was 97% for sustained episodes of 5 minutes or longer.73 Some devices have incorporated newer detection algorithms that include pattern recognition to facilitate detection of AT/AF and minimize inappropriate detection caused by FFRW oversensing.72,73 Such algorithms have high sensitivity (>95%) and specificity for AT/AF detection.72–75
Pacing Mode in Prevention of Atrial Fibrillation
Several prospective randomized clinical trials have reported that atrial or dual-chamber pacing prevents paroxysmal and permanent AF in patients with symptomatic bradycardia as the primary indication for cardiac pacing. Danish investigators reported a 46% relative risk reduction for the development of AF in the atrial pacing group (P = .012).48 The CTOPP study reported an 18% risk reduction in the development of AF (P = .05) in patients randomized to atrial-based versus ventricular pacing.50,76 This effect was sustained over longer-term follow-up (20% risk reduction at 6 years; P = .009).51 Patients with a structurally normal heart were most likely to derive benefit from atrial-based pacing.76 Although a retrospective subgroup analysis in CTOPP suggested that pacemaker-dependent patients were most likely to benefit from physiologic pacing for prevention of AF, this effect was not confirmed over long-term follow-up.51 The PASE investigators reported a 32% reduction in AF in patients with SND as the primary indication for pacing who were randomized to the dual-chamber pacing mode versus the ventricular pacing mode (P = .06).49 MOST reported a 21% relative risk reduction in the development of AF (P = .008) and a 56% relative risk reduction in the development of permanent AF (P < .001) in patients randomized to dual-chamber versus ventricular pacing52 (Fig. 13-8).
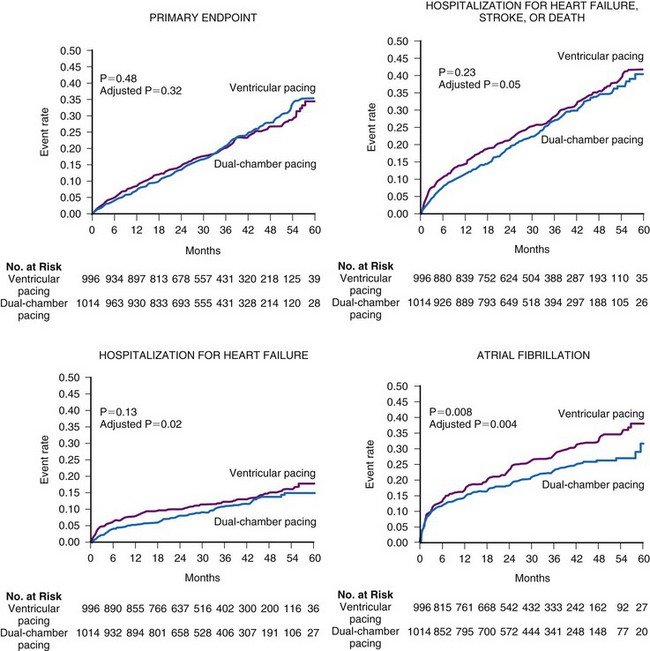
Figure 13-8 Rates of clinical events according to the mode of pacing in the MOST trial.
(From Lamas GA, Lee KL, Sweeney MO, et al: Mode Selection Trial in Sinus Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus node dysfunction. N Engl J Med 346:1854-1862, 2002.)
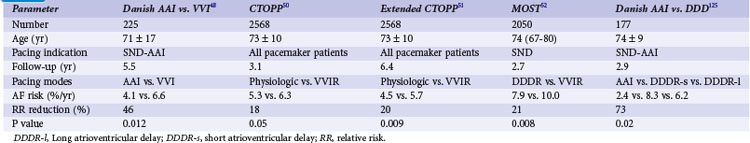
Table 13-4 summarizes the results of these clinical trials. In contrast, the United Kingdom Pacing and Cardiovascular Events (UKPACE) trial investigators, who randomized 2021 patients age 70 and older with high-grade AV block to dual-chamber or ventricular pacing, did not observe a benefit of dual-chamber pacing for AF.77 These data suggest that the primary benefit of dual-chamber pacing for prevention of AF occurs in patients with SND.
In the CTOPP population, the number needed to treat (NNT) to prevent any AF over 10 years (ideal longevity of dual-chamber pacemaker) was nine patients.58 In the MOST population, NNT to prevent permanent AF in patients with SND over 3 years was nine patients. The cost differential of dual-chamber pacing compared with ventricular pacing in CTOPP was $2500.78 The additional cost of dual-chamber pacing is approximately $1/day. AF is frequently unrecognized in the pacemaker population; anticoagulation is often underutilized; and class I-III antiarrhythmic drug therapy for prevention of AF may be proarrhythmic. Accordingly, atrial or dual-chamber pacing may be a cost-effective therapy to prevent a condition that causes substantial morbidity.78,79 However, to date, no study has shown that prevention of AF in this patient population translates into a substantial clinical benefit, whether improved quality of life or functional capacity or reduced hospitalization or health care utilization.
Pacing Algorithms in Prevention of Atrial Fibrillation
Over the past decade, a number of clinical studies have evaluated the role of atrial pacing algorithms for prevention of atrial fibrillation.58,59,80 Initial studies investigated the efficacy of atrial overdrive pacing achieved by programming a higher base pacing rate on suppression of AF.81,82 Subsequently, selective pacing algorithms were designed to target the triggers and substrate for AF, including continuous pacing rather than fixed-rate atrial overdrive, algorithms to prevent pauses after atrial premature beats or exercise, transient overdrive pacing after detection of premature atrial contractions (PACs) or after termination of an episode of AF, and atrial ATP for termination of atrial flutter that transitions into AF.58,60,61 Although small studies suggested promise of one or more of these algorithms for suppression of AF, larger randomized clinical trials have failed to demonstrate a major benefit of these therapies for prevention of AF compared to control groups with fixed-rate or rate-responsive pacing.
To test the hypothesis that atrial pacing might prevent paroxysmal AF in patients without symptomatic bradycardia as a primary indication for pacing, the Atrial Pacing Peri-Ablation for the Prevention of AF (PA3) study randomized 97 patients with frequent paroxysmal AF being considered for AV junction ablation to a trial of atrial pacing versus no pacing.81 The time to first recurrence of AF and total AF burden documented over 3-month follow-up were similar in the atrial pacing group (1.9 days; 95% CI = 0.8 to 4.6) compared to the no pacing group (4.2 days; 95% CI = 1.8 to 9.5; P = NS). In Phase II the PA3 trial tested the hypothesis that atrial pacing versus AV synchrony would prevent AF.82 After AV junction ablation, 76 patients were randomized to DDDR versus VDD pacing. The time to first recurrence of sustained AF and total AF burden over time were similar in the DDDR (0.37 day; 95% CI = 0.1 to 1.3) and the VDD (0.5 day; 95% CI, 0.2 to 1.7; P = NS) pacing groups. Furthermore, AF burden increased progressively over time, and 42% of the study population had developed permanent AF within 1 year after ablation. Patients maintained on constant antiarrhythmic drug therapy throughout the PA3 study developed significant increases in AF burden and were more likely to develop permanent AF very early after AV junction ablation than patients with deferred ablation.83
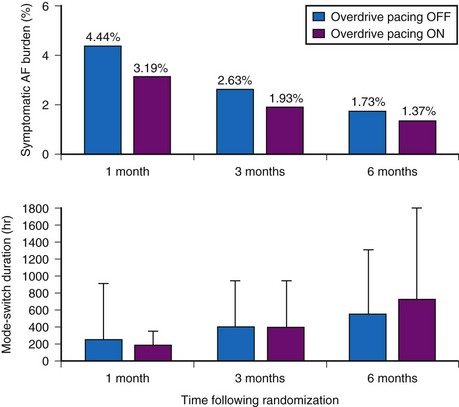
The dynamic atrial overdrive (DAO) pacing algorithm was evaluated in the Atrial Dynamic Overdrive Pacing Trial (ADOPT).84 Investigators randomized 399 patients with SND and paroxysmal AF to a trial of DDDR pacing versus DDDR pacing plus the overdrive atrial pacing algorithm. Patients were followed at 1, 3, and 6 months after pacemaker insertion. The primary study outcome was symptomatic AF burden, defined as percentage of days in symptomatic AF. Both groups experienced a substantial reduction in symptomatic AF over time. In addition, a modest but statistically significant reduction was found in symptomatic AF associated with the DAO algorithm during follow-up (Fig. 13-9, upper panel). However, the absolute risk reduction diminished over time: 1.25% at 1 month versus 0.36% at 6 months. Moreover, overall AF burden calculated from the mode-switch diagnostics was similar between the two groups and indeed, increased over time, suggesting that the overdrive pacing algorithm had no effect on total AF burden over time (Fig. 13-9, lower panel).
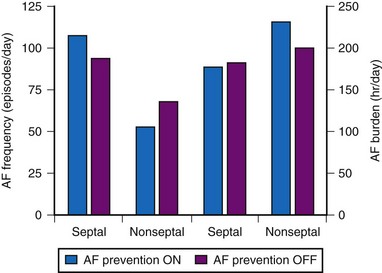
Other studies have not demonstrated a significant impact of DAO pacing for prevention of AF. The Atrial Septal Pacing Clinical Efficacy Trial (ASPECT) randomized 298 patients with paroxysmal AF and associated symptomatic bradycardia to conventional DDDR pacing or DDDR pacing plus three additional atrial pacing algorithms specifically designed for prevention of AF at atrial-septal or right atrial appendage pacing sites.85 After 3 months of treatment, patients were crossed-over to the alternate pacing strategy and followed for an additional 3 months. AF burden, determined from the diagnostic counters (in pacemaker measured as % time in AF) was the primary study outcome. The three AF prevention algorithms (atrial pacing preference, atrial rate stabilization, and “post mode switch overdrive” pacing) did not reduce AF burden or AF frequency despite suppression of atrial premature beat frequency (Fig. 13-10).
The AFTherapy Study randomized 372 patients with drug-refractory paroxysmal AF to dual-chamber pacing programmed to single-rate or rate-responsive pacing at lower rates of either 70 or 85 bpm or to a control group with single-rate pacing at 40 bpm.60 In the subsequent preventive pacing phase, patients were randomized to pacing at a lower rate of 70 bpm with or without programming of four preventive pacing algorithms. The primary endpoint was AF burden, with secondary endpoints of time to first AF episode and averaged sinus rhythm duration. Substantial data were excluded from the analysis because of atrial-sensing issues, predominantly oversensing, resulting in inappropriate classification of events as AF. In the conventional pacing phase, no significant differences in AF burden were found between the various lower rates and the control group or between single-rate and rate-responsive pacing. In the second phase, patients receiving preventive pacing therapies had a similar AF burden (median, 0 hr/day) compared with patients receiving conventional pacing (0.18 hr/day; P = 0.47).
In the Study of Atrial Fibrillation Reduction (SAFARI) trial, 240 patients with qualifying AF 4 months after pacemaker implantation were randomized to a suite of six pacing prevention therapies programmed on or off and followed for 6 months.61 A modest but statistically significant decrease in AF burden (median, 0.08 hr/day) was seen in the group randomized to AF prevention therapies on
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree