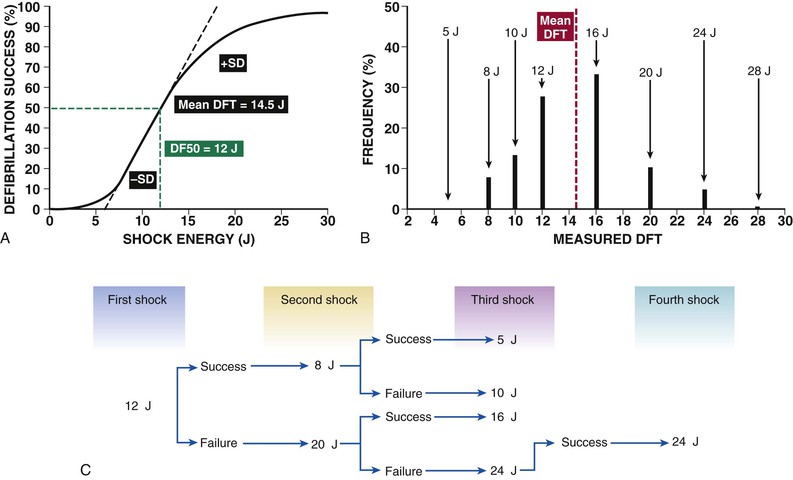
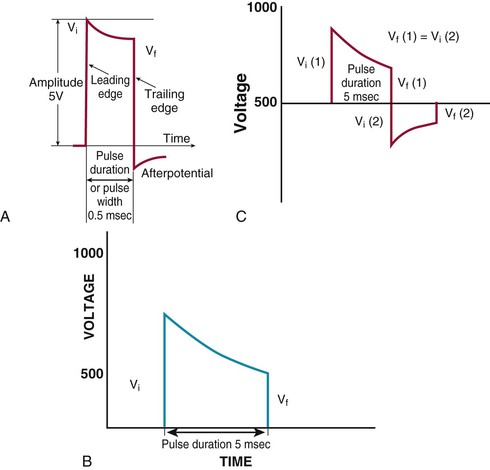
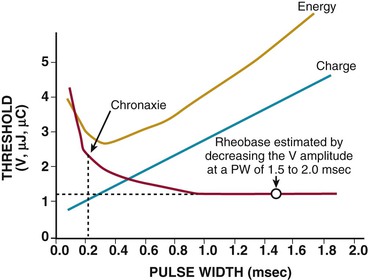
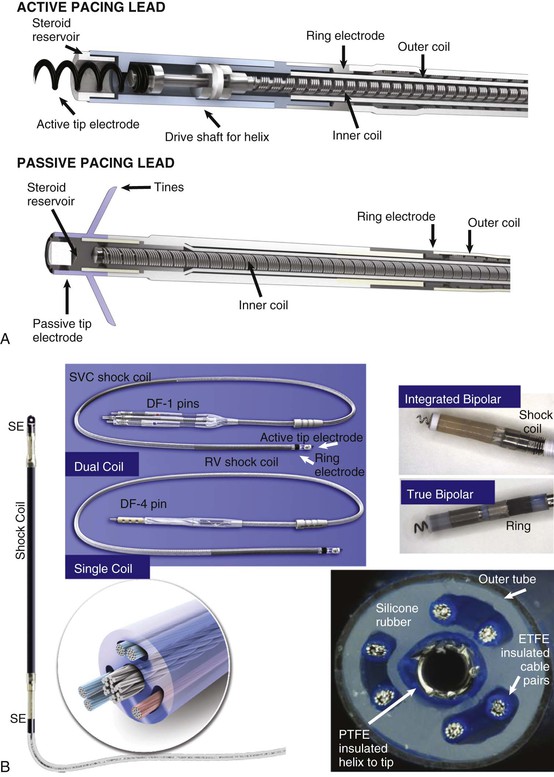
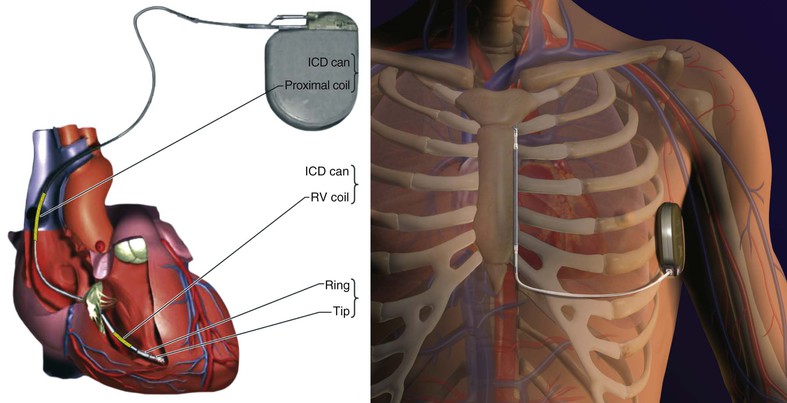
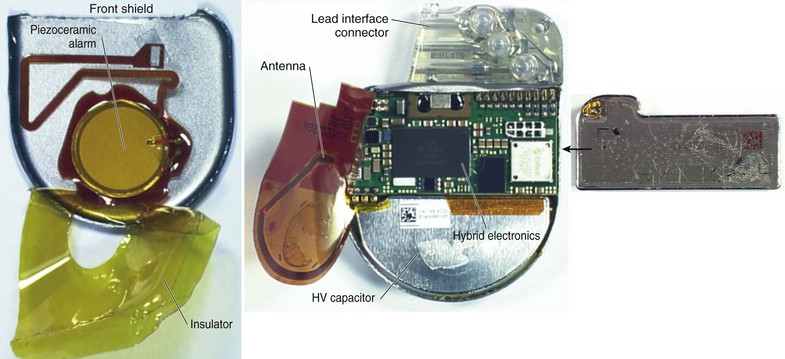
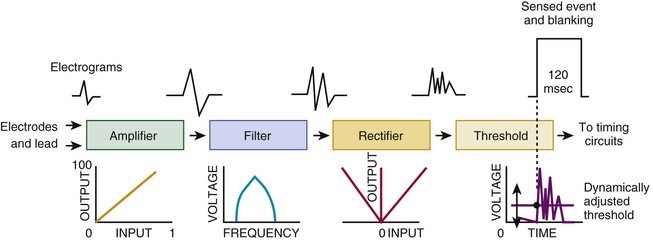
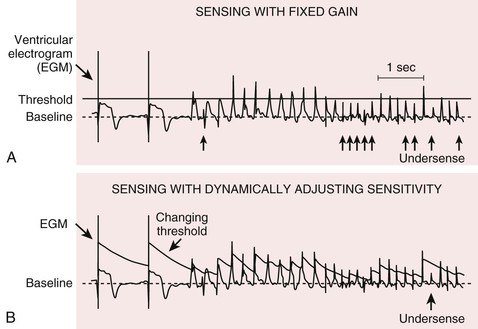
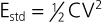Charles D. Swerdlow, Paul J. Wang, Douglas P. Zipes Electrical therapy for cardiac arrhythmias includes low-voltage pacing pulses, which are used to treat bradycardia or to provide antitachycardia pacing (ATP) for termination of reentrant tachycardias, and high-voltage shock pulses, which are used to defibrillate atrial fibrillation (AF) or ventricular fibrillation (VF) or to cardiovert ventricular tachycardia (VT). An applied electrical stimulus interacts with cardiac electrical activity via its resultant electrical field, which is proportional to the spatial derivative of the voltage applied (local rate of change with respect to the distance derivative). The response of the heart is mediated by the passive and active (ion channel) properties of cell membranes, by the properties of electrical connections between cardiac cells, and possibly by direct intracellular electrical effects. Heart rate and stroke volume are the two determinants of cardiac output, which increases fivefold to sixfold to meet the metabolic demands from rest to peak exercise. The ability of the heart rate to increase during exertion is termed chronotropic competence. It plays a particularly large role as exertion approaches its peak. Atrial filling of the left ventricle occurs throughout diastole as long as the mitral valve remains open, beginning with the early diastolic filling phase. At the end of diastole, immediately before the onset of systole, the atria contract, which results in a bolus of blood that contributes appreciably to ventricular stroke volume. Maximizing the atrial contribution to cardiac output requires optimal timing of electrical activation of the atria before the onset of ventricular contraction. The coordination of atrial and ventricular electrical activation and mechanical contraction is called atrioventricular (AV) synchrony. The presence of AV synchrony may increase cardiac output by 25% to 30%. Patients with impaired diastolic function or impaired systolic function are most dependent on atrial transport. Any circumstance that prevents appropriate timing of atrial and ventricular contraction can result in impaired AV synchrony along with its hemodynamic consequences. The most hemodynamically disadvantageous AV timing relationship occurs during ventricular pacing with retrograde (ventriculoatrial [VA]) conduction, which results in reverse (VA) synchrony and atrial contraction while the AV valves are closed. Pacemaker syndrome can occur if retrograde conduction is present during single-chamber ventricular pacing or during dual-chamber pacing with loss of atrial pacing or with unreliable sensing. Patients with long PR intervals can exhibit several causes of impaired mechanical dyssynchrony (despite electrical synchrony), depending on the degree of PR prolongation. If the PR interval is extremely long such that the preceding P wave occurs during the preceding ventricular systole, atrial contraction occurs when the mitral valve is closed, a situation akin to ventricular pacing with retrograde conduction but without retrograde atrial activation. If the PR interval is slightly shorter, atrial contraction occurs after the mitral valve has opened but before much of the passive atrial contribution to ventricular filling has been completed. As a result, there may be diastolic mitral regurgitation because of backward flow from the left ventricle to the left atrium while the mitral valve remains open before ventricular systole has begun. If the PR interval is too short, the contribution of atrial contraction is insufficient because the mitral valve closes before atrial systole is complete. Pacemaker syndrome refers to the constellation of symptoms caused by loss of mechanical AV synchrony. As noted, it may occur with AV dissociation or with 1 : 1 AV association that results in an adverse sequence of ventricular and atrial contraction. Studies have examined the clinical consequences of not having AV synchrony. In the MOST study, patients with sinus node disease were randomly assigned to DDD versus VVI pacing. The study demonstrated a lower incidence of AF and heart failure in the DDD pacing arm.1 In patients with impaired AV conduction, DDD pacing delivers right ventricular pacing to ensure that the AV interval is in the physiologic range. However, right ventricular pacing results in intraventricular asynchrony, which has adverse hemodynamic effects (see Chapter 26). In patients with underlying left ventricular dysfunction, right ventricular pacing increases the incidence of heart failure and AF.2 Although no maximal PR interval has been established, some clinicians use a “cutoff” of approximately 350 to 400 milliseconds. In patients with intact AV conduction but a long PR interval, there may be a hemodynamic “trade-off” between having optimal AV timing and accepting the impaired hemodynamics of right ventricular pacing. Pacing algorithms to avoid unnecessary right ventricular pacing in patients with normal intraventricular conduction are discussed later under Pacing Modes. The American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines for device-based therapy for cardiac rhythm abnormalities were updated in 2008.3 The Guidelines section of this chapter provides guidelines that apply to pacemakers and ICDs. Guidelines for cardiac resynchronization devices are presented in Chapter 26. The main indications for permanent pacing are to relieve or prevent symptomatic bradycardia. They are supported by strong expert consensus but were developed before the era of randomized controlled trials. The strongest indications are related to relief of symptoms confirmed to be caused by bradycardia. Pacing is also indicated for patients who have documented asymptomatic bradycardia and for those with symptoms consistent with bradycardia but no documentation of bradycardia during symptoms, provided that alternative causes of the symptoms have been excluded and the symptoms are sufficiently serious. Pacing is indicated to prevent symptomatic bradycardia in asymptomatic patients if the risk for rapid progression to serious symptoms is high. This indication is applied most commonly to patients with advanced disease of the His-Purkinje system who are at risk for abrupt, high-grade AV block without an adequate escape rhythm. ICDs are indicated for prevention of sudden death from VT/VF either as “secondary prevention” in patients who have been resuscitated from sustained VT/VF or as “primary prevention” in patients without arrhythmic symptoms who are judged to be at sufficient risk for VT/VF. ICDs are the treatment of choice for secondary prevention of VT/VF, provided that patients remain at risk for recurrence of VT/VF and have sufficient life expectancy and quality of life to justify implantation. The strong consensus on the use of ICDs for secondary prevention is based on multiple, randomized controlled trials that compared antiarrhythmic drugs with ICDs, including the AVID (Antiarrhythmics Versus Implantable Defibrillators) study.4 Presently, more than 80% of ICDs are implanted for primary prevention. The MADIT II5 and SCD-HeFT6 randomized controlled trials demonstrated absolute mortality reductions of 5% to 7% over a period of 2 to 4 years in high-risk patients with ischemic or nonischemic cardiomyopathy. High-risk patients are identified primarily by heart failure class and a left ventricular ejection fraction of 30% to 35% or lower. Guidelines based on more limited evidence identify subgroups of high-risk patients with less common diseases, including hypertrophic cardiomyopathy (see Chapter 66) and ion channelopathies (see Chapters 32, 35, and 37). Near-uniform consensus supports secondary-prevention guidelines, but support for primary-prevention guidelines is less consistent. On average, patients who receive ICDs in clinical practice are older and have more serious comorbid conditions, including diabetes and renal failure, than do patients in the foundational clinical trials. Retrospective analyses indicate that ICDs (excluding cardiac resynchronization devices) do not prolong life in identifiable subgroups of primary-prevention patients with extensive comorbidity. Additionally, approximately 15 to 20 primary-prevention ICDs must be implanted in asymptomatic patients to save one life. Not surprisingly, patients vary in their willingness to accept implantation of ICDs to treat statistical risk. A recent expert consensus document provides guidelines for the selection of single- versus dual-chamber pacemakers and summarizes the supporting clinical evidence.7 In patients with sinus node disease, multiple randomized controlled trials have demonstrated that dual-chamber pacing is associated with a lower incidence of AF and pacemaker syndrome than single-chamber ventricular pacing is. These studies reported inconsistent results regarding reduction in heart failure, stroke, and quality of life. Dual-chamber pacemakers should be programmed to minimize right ventricular pacing in patients with intact AV conduction. Rate-adaptive pacing is recommended for patients with significant symptomatic chronotropic incompetence who demonstrate improvement in symptoms after rate-adaptive pacing is programmed. Single-chamber atrial pacing is not generally recommended because many patients with sinus node disease are at risk for AV block, but it may be considered in patients with normal AV and ventricular conduction. Dual-chamber pacing is recommended instead of single-chamber ventricular pacing in patients with these blocks based on expert consensus. However, randomized controlled trials performed exclusively or primarily in elderly, sedentary patients did not support the superiority of dual-chamber pacing for major endpoints other than pacemaker syndrome (e.g., AF, stroke, heart failure). Early, acute randomized studies demonstrated that dual-chamber pacing improves exercise tolerance when compared with fixed-rate ventricular pacing, but benefit over rate-adaptive ventricular pacing has been inconsistent. Thus single-chamber ventricular pacing is an acceptable alternative to dual-chamber pacing in patients with AV block who have clinical conditions that limit the benefits of dual-chamber pacing (e.g. sedentary lifestyle) and in those in whom technical issues such as limitations in vascular access preclude or increase the risk associated with inserting an atrial lead. Presently, expert consensus does not provide guidelines for the selection of single- versus dual-chamber ICDs. Dual-chamber ICDs provide dual-chamber pacing, diagnostics for AF, and discriminators of supraventricular tachycardia (SVT) and VT that are not available in single-chamber ICDs, and their stored EGMs provide higher diagnostic accuracy than single-chamber ones do. Disadvantages of dual-chamber ICDs include higher cost, atrial lead complications, and decreased longevity. Dual-chamber pacing modes that minimize ventricular pacing are important in ICD patients because of their high prevalence of left ventricular dysfunction, and they reduce the risk for heart failure as a result of obligatory right ventricular pacing in ICD patients. Randomized, controlled studies and a meta-analysis have shown a modest benefit of dual-chamber over single-chamber pacing for discrimination of SVT and VT in secondary-prevention patients in whom monomorphic VT occurs at rates that overlap the ventricular rates in SVT or sinus tachycardia. They show no benefit in primary-prevention patients and are unlikely to benefit secondary-prevention patients whose only arrhythmia is VF. Presently, there is no consensus regarding the use of single- versus dual-chamber ICDs, except in patients who require dual-chamber pacing. The most commonly used nomenclature for pacing modes involves a four-letter code (Table 36-1). The first letter stands for the chamber paced: A for atrium, V for ventricle, and D for dual—both atrium and ventricle. The second letter stands for the chamber sensed: A for atrium, V for ventricle, and D for dual—both atrium and ventricle. The third letter is the function: I for inhibition, T for triggered, and D for dual tracking of atrial activity while inhibited by ventricular activity. The fourth letter is R for rate adaptive. The letter “O” indicates absence of that function. Often it is easier to analyze pacing modes in terms of their associated time intervals (or “periods”) measured in milliseconds than in terms of their rate measured in beats/min. One advantage of using intervals is that they can be added. A second advantage is that intervals accurately describe a cardiac rhythm that varies from beat to beat whereas rate refers to an average value if the rhythm is irregular. Because 1 minute is equivalent to 60,000 milliseconds, the interval in milliseconds corresponding to a rate in beats/min can be determined by dividing the rate into 60,000 (Table e36-2 The VVI mode is the basic single-chamber ventricular pacing mode; it allows pacing to occur when the ventricular rate slows below the programmed lower rate limit (Fig. 36-7). The interval corresponding to the lower rate limit is the ventricular pacing interval. Usually, this is equal to the interval between a sensed ventricular event and the next paced ventricular event, referred to as the “ventricular escape interval.” There is no atrial sensing, so AV synchrony is not preserved. This mode is indicated for patients with permanent AF. The AAI mode is the corresponding single-chamber atrial pacing mode (Fig. 36-8). It is appropriate for patients with sinus node dysfunction and normal AV conduction. Because it does not provide ventricular pacing, it should not be used in patients at risk for AV block. The DDD pacing mode is most commonly used in the patients whose rhythm is not permanent AF (Fig. 36-9). In this mode the atrial rate cannot go lower than the programmed lower rate. A programmed AV delay is the maximum time permitted from an atrial event to a ventricular event. If a spontaneous ventricular event does not occur by the time that the AV delay elapses, a ventricular paced event occurs. In the setting of AV block, all ventricular events are paced. A special characteristic of the DDD pacing mode is the ability to “track” intrinsic atrial activity to maintain AV synchrony. The DDD mode has an upper rate limit, the maximum rate that intrinsic atrial activity will be tracked. The maximum rate is selected to exceed the maximum sinus rate that the patient is capable of achieving. The upper rate limit is predominantly of importance to prevent tracking of rapid atrial activity in spontaneous atrial arrhythmias such as AF. At slow ventricular rates, most of the cardiac cycle constitutes a sensing alert period during which sensed events are used for both pacemaker timing cycles and detection of tachyarrhythmias. After each sensed event the sense amplifier is turned off for a short blanking period (20 to 250 milliseconds) to prevent multiple sensed events during a single cardiac depolarization. Following each blanking period there is a refractory period during which events may be sensed for tachyarrhythmia detection algorithms but do usually not alter pacemaker timing cycles (Fig. 36-10; also see Figs. 36-5 and 36-7 to 36-9). The blanking and refractory periods in the ventricle after atrial sensed or paced events and in the atrium after ventricular sensed or paced events are called cross-chamber blanking and refractory periods. Cross-chamber blanking periods reduce oversensing of the pacing artifact after a paced event in the opposite chamber. The postventricular atrial blanking period (after ventricular events) reduces atrial oversensing of ventricular pacing stimuli and far-field R waves, which may result in incorrect diagnosis of an atrial tachyarrhythmia. ICDs typically have shorter blanking and refractory periods than pacemakers do so that short cardiac cycles can be sensed reliably. In the DDD mode, there is a special refractory period called the postventricular atrial refractory period (PVARP) that starts with any ventricular event and defines a period on the atrial channel during which a spontaneous atrial event is not tracked. The PVARP is especially important in patients with retrograde conduction. If the PVARP is too short, a premature ventricular beat may be conducted retrogradely, sensed on the atrial channel, and tracked, thereby resulting in a second (paced) ventricular beat that can be conducted retrogradely. This repetitive sequence of ventricular pacing, retrograde conduction, and atrial tracking of the retrogradely conducted beat represents one form of pacemaker-mediated tachycardia (Fig. e36-4 The PVARP has important implications regarding upper rate behavior. Because the ventricular rate cannot exceed the programmed upper rate limit, an algorithm is needed to determine how the ventricular pacing rate should be adjusted in patients with AV block when the sinus rate exceeds the upper rate limit. All pacemakers share the algorithm for extending the AV delay when the sinus rate exceeds the upper rate limit so that the ventricular pacing rate is at the programmed upper rate. Because the sinus rate is faster than the ventricular pacing rate, P waves will occur progressively earlier after each successive ventricular paced beat. Eventually the sinus beat falls within the PVARP and is no longer tracked. This progressive prolongation of the AV delay until a sinus beat times in the PVARP and is not followed by a paced ventricular beat is often called “pseudo–AV Wenckebach.” Unlike biologic Wenckebach, the ventricular rate remains constant at the upper rate limit. If the sinus rate increases further so that every other P wave will time within the PVARP, the pacemaker will track every other P wave and thereby result in 2 : 1 atrial tracking. Consider the slowest atrial rate that results in 2 : 1 atrial tracking. The tracked P wave will be followed by a ventricular paced beat at the programmed AV interval, and the ventricular beat will be followed by the nontracked P wave exactly by the duration of the PVARP. Thus the time from the tracked P wave to the nontracked P wave equals the sum of the programmed AV delay and the PVARP, which is called the total atrial refractory period (TARP). Such 2 : 1 tracking results in an abrupt decrease in the ventricular rate (Fig. 36-11) and often causes exertional intolerance if it occurs during exercise-induced sinus tachycardia. Consequently, it is important to keep the TARP below the maximum sinus rate during exercise. The DDI pacing mode is similar to the DDD mode but lacks atrial tracking and therefore an upper rate limit. It can be used in patients with sinus bradycardia, with or without intact AV conduction. Today it is rarely programmed unless atrial sensing problems prevent reliable DDD pacing. The VDD pacing mode is suitable for patients with intact sinus node function and AV block because only the ventricular chamber is paced but sensing occurs in both the atrium and ventricle. Intrinsic sinus beats are tracked as in the DDD mode. A special lead with floating atrial electrodes for sensing and standard ventricular electrodes for pacing and sensing permits single-lead VDD pacing. Rate-adaptive pacing adjusts the pacing rate to the metabolic demands of the body. A sensor located in the pacemaker generator or lead monitors a signal that may indicate the need for an increased heart rate. Commonly used sensors monitor body motion (accelerometer), respiration (minute ventilation), or cardiac motion (endocardial acceleration), and each has specific advantages and limitations. Algorithms translate the sensor values to a pacing rate. Most algorithms have programmable parameters to achieve the optimal heart rate for the body’s metabolic needs. Automatic mode switching in the DDD pacing mode initiates a temporary change in mode to a nontracking one (usually DDI or DDIR) during paroxysmal atrial tachyarrhythmias. This prevents the adverse consequences of rapid ventricular pacing as a result of tracking nonphysiologic high atrial rates. Most mode-switching algorithms use the atrial rate as an indicator for the onset of an atrial tachyarrhythmia. When the atrial rhythm again meets the defined criteria for a physiologic rhythm, the mode switches back to an atrial tracking mode (Fig. 36-12). Because only a small proportion of patients with sinus node dysfunction receive a single-chamber AAIR pacemaker, strategies to minimize right ventricular pacing are important to reduce the adverse clinical effects of unnecessary right ventricular pacing and to prolong generator longevity. One common strategy in patients with AV conduction is a variation on AAIR pacing with back-up ventricular pacing. Such algorithms perform in the AAIR pacing mode when AV block is not present but switch automatically to the DDDR mode when AV block is detected. This algorithm also checks periodically to determine whether AV conduction has resumed and returns to AAIR pacing when conduction resumes. The advantage of this commonly used approach is that it can be tolerant of occasional single beats of AV block without resorting to consistent ventricular pacing but provides ventricular pacing with a physiologic AV interval. These algorithms are programmed commonly, but they may mimic intermittent failure of ventricular pacing for a single beat. They may be differentiated from oversensing in that ventricular tracking always resumes after a blocked P wave (Fig. 36-13). An alternative strategy is to prolong the AV interval to allow intrinsic AV conduction. If intrinsic ventricular activation is detected, the AV delay remains extended. If ventricular activation is not detected within a given AV delay range, ventricular pacing resumes. This prevents single beats of AV block, but it usually results in a higher percentage of ventricular paced beats. Periodic extension of the AV delay to detect intrinsic ventricular activation is termed “positive search AV hysteresis.” Either strategy may result in the extremely long AV delays that cause pacemaker syndrome. Pacemakers and ICDs also incorporate algorithms to optimize function based on sensing. These include algorithms to prevent inhibition during oversensing and loss of pacemaker capture. Ventricular safety pacing prevents inappropriate pacemaker inhibition caused by ventricular oversensing of atrial pacing stimuli (crosstalk; Fig. e36-5
Pacemakers and Implantable Cardioverter-Defibrillators
Background: Cardiac Electrical Stimulation
Hemodynamics Related to Pacing
Chronotropic Response
Atrioventricular Synchrony
Adverse Consequences of Right Ventricular Pacing
Indications and Device Selection
Indications: Pacemakers
Indications: Implantable Cardioverter-Defibrillators
Single- Versus Dual-Chamber Pacemakers and Implantable Cardioverter-Defibrillators
Single- Versus Dual-Chamber Pacemakers
Sinus Node Disease
Atrioventricular Block and Bifascicular/Trifascicular Block
Single- Versus Dual-Chamber Implantable Cardioverter-Defibrillators
Pacing Modes, Timing Cycles, Blanking, and Refractory Periods
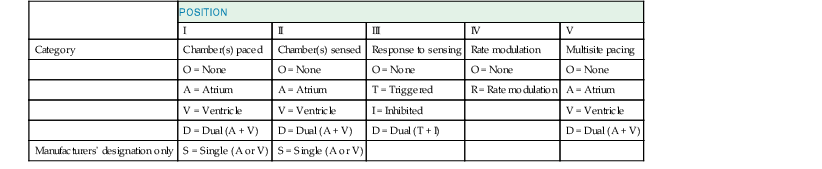
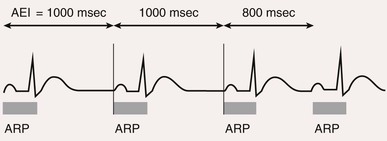
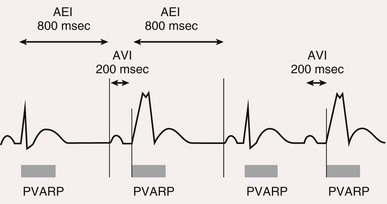
Pacing Modes
![]() ).
).
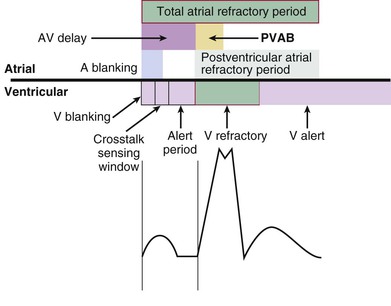
Blanking and Refractory Periods
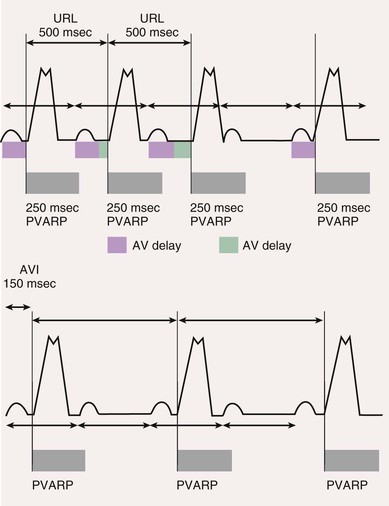
Definitions
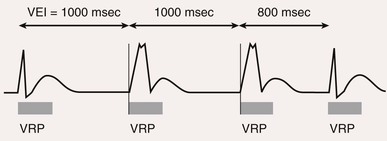
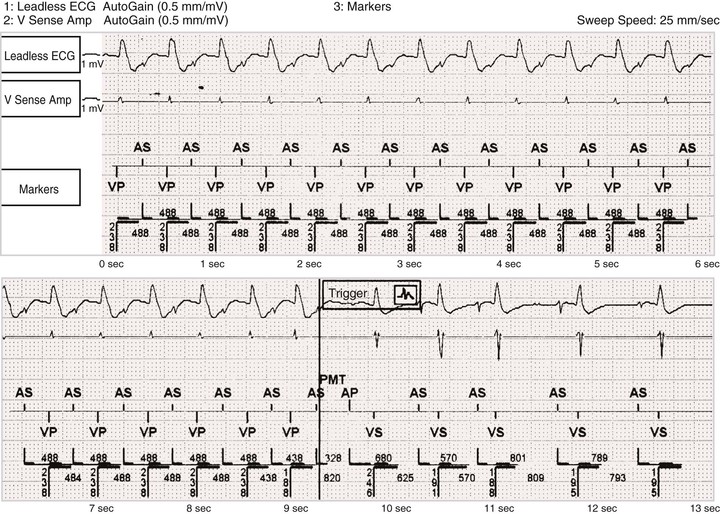
Postventricular Atrial Refractory Period
![]() ).
).
Rate-Adaptive Pacing
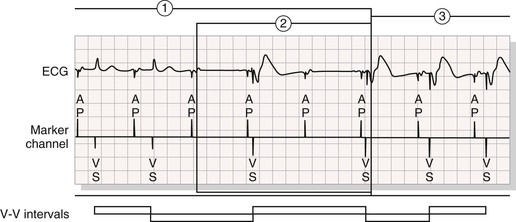
Automatic Mode Switching
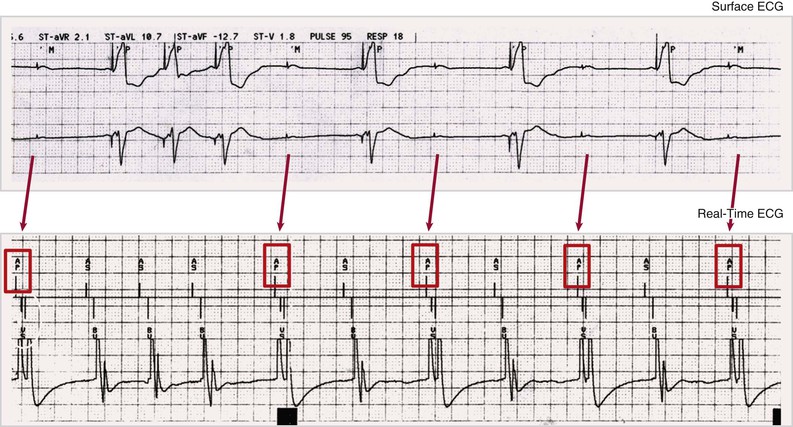
Pacing Algorithms to Avoid Unnecessary Right Ventricular Pacing
Automatic Optimization of Other Pacemaker Function Based on Sensing
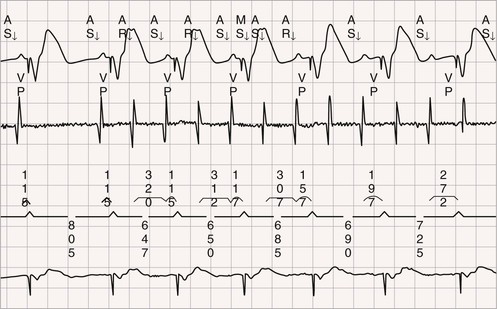
![]() ). Safety pacing may be identified on ECGs by noting a shorter than programmed AV delay, usually 80 to 130 milliseconds. Noise reversion to fixed-rate asynchronous pacing prevents pacemaker inhibition during continuous ventricular oversensing, including that occurring during electromagnetic interference from sources such as electrocautery. Automatic assessment of the pacing capture threshold is performed by closed-loop feedback algorithms that periodically test capture and adjust the output based on test results. This feature permits use of an output that is just sufficient to achieve capture and results in safety, as well as conservation of battery energy.
). Safety pacing may be identified on ECGs by noting a shorter than programmed AV delay, usually 80 to 130 milliseconds. Noise reversion to fixed-rate asynchronous pacing prevents pacemaker inhibition during continuous ventricular oversensing, including that occurring during electromagnetic interference from sources such as electrocautery. Automatic assessment of the pacing capture threshold is performed by closed-loop feedback algorithms that periodically test capture and adjust the output based on test results. This feature permits use of an output that is just sufficient to achieve capture and results in safety, as well as conservation of battery energy.
Detection of Ventricular Tachycardia and Fibrillation in Implantable Cardioverter-Defibrillators
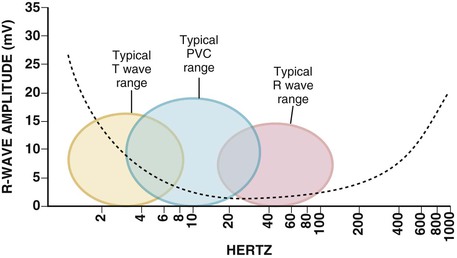
Rate, Duration, and Detection Zones
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pacemakers and Implantable Cardioverter-Defibrillators
36