Fig. 11.1
Surface landmarks of the chest. The craniocaudal border of the chest is therefore defined as the clavicle to the costal margin. Posteriorly, the inferior border lies at the inferior margin of the scapula
There is crossover between the superior border of the abdomen and the inferior border of the chest. Superiorly, the border of the abdomen is defined as the nipple line. Wounds existing in the area between the nipple (or inframammary fold for females) and the costal margin are considered thoracoabdominal and warrant both a thoracic and abdominal evaluation.
11.3.2 The “Box”
The majority of stab wounds (approximately 80 %) causing injury to the heart enter through the precordium. This area, often referred to as the “box,” is defined as an area bounded superiorly by the clavicles, laterally by the nipples, and inferiorly by the xiphoid process (Fig. 11.2). This four-sided area is extended posteriorly to the back. Injuries to this area and missiles with trajectories passing through this area mandate the proper cardiac evaluation as defined later in this section. It should be noted that the majority of gunshot wounds causing cardiac injury do not enter through this anatomic area, so suspicion based on presumed trajectory and clinical presentation is necessary for accurate and timely diagnosis [2]. The standard array of chest incisions are depicted in Fig. 11.3.
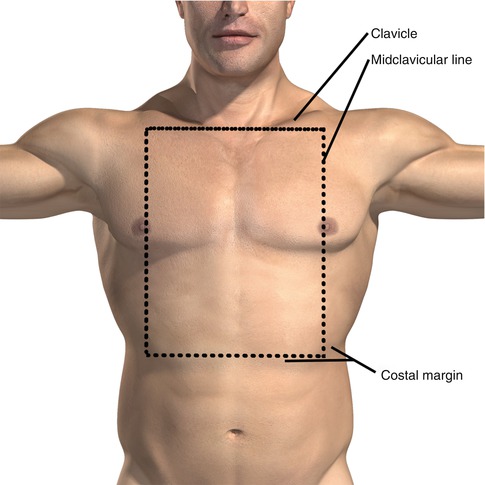
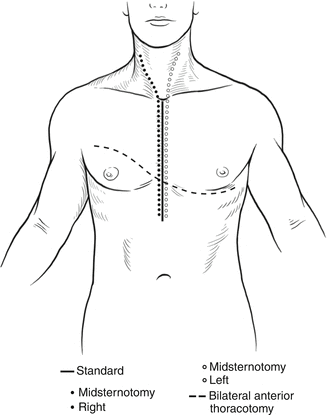

Fig. 11.2
The box. The majority of stab wounds (around 80 %) causing injury to the heart enter through the precordium. This area, often referred to as the “box,” is defined as an area bounded superiorly by the clavicles, laterally by the nipples, and inferiorly by the xiphoid process

Fig. 11.3
Chest incisions
11.4 Anatomic Relationships
11.4.1 Heart
As stated above, the injuries to the precordium (especially stab wounds) are associated with a higher incidence of cardiac injury. Externally, the superior border of the heart lies approximately at the second intercostal space bordered laterally by points approximately 2 cm lateral to either side of the sternum. The inferior border, formed by the right ventricle, can be thought of as connecting the point of maximal impulse to the right sixth costal cartilage. Connecting the margins of the inferior and superior borders estimates the lateral borders of the heart. The left ventricle creates the left lateral border of the heart.
11.4.2 Arch Vessels
Arising from the aortic valve in the fibrous trigone of the heart, the short ascending aorta gives rise to the aortic arch. The arch is oriented primarily in the anterior-posterior plane with some leftward deviation beginning in the midline and ending up just to the left of the vertebral column. The three great vessels arise from the top of the aortic arch. Beginning proximally, they are the right brachiocephalic, left common carotid, and left subclavian arteries. The great vessels, in combination with the ligamentum arteriosum, fix the aorta to the chest. Over 90 % of aortic disruptions seen in blunt trauma occur in this area due to this fixation point.
11.4.3 Subclavian
The left subclavian artery arises reliably from the aortic arch. The right side typically arises from the brachiocephalic artery but may, rarely, arise directly from the aortic arch or from a common trunk shared by the right common carotid artery. Bilaterally, the arteries are divided into three parts by the anterior scalene muscles. The medial portion gives off the vertebral artery and thyrocervical trunk. The portion deep to the muscle gives rise to the costocervical artery. Laterally, the artery is without branches becoming the axillary artery as the artery crosses the medial portion of the clavicle.
11.5 Assessment and Initial Management
All patients with suspected injuries to the thorax should be evaluated and resuscitated according to the principles of the American College of Surgery-Committee On Trauma (ACS-COT) Advanced Trauma Life Support (ATLS) guidelines and protocols [3]. The primary survey will address immediate threats to life and will aid in the triage and further management of all injured patients. Prior to arrival, there should be communication with the transporting emergency services team in order to ascertain the hemodynamic status of the patient. Patients may be thought of as hemodynamically normal or abnormal or have lost signs of life and are actively undergoing cardiopulmonary resuscitation, the latter potentially qualifying for a resuscitative (ED) thoracotomy.
Patients should undergo a standard airway assessment as outlined in ATLS. The decision to secure a patient’s airway is multifactorial and takes into consideration the patient’s physiologic presentation as well as other injuries. One pitfall is failure to recognize a tension pneumothorax in a patient presenting with respiratory distress. Patients presenting with respiratory distress with decreased breath sounds or a hyperinflated hemithorax along with hypotension (assessment of jugular venous distension may be compromised in the face of hypovolemia) should have their chest decompressed prior to attempting intubation. The reason is threefold: respiratory distress may resolve following appropriate chest decompression alleviating the need for intubation, intubation of a patient with tension physiology is difficult due to shifting anatomy, and induction agents may worsen the patient’s shock state and hasten their cardiovascular collapse.
The importance of a properly inserted tube thoracostomy in the setting of thoracic trauma cannot be overstated. The majority of patients presenting with thoracic trauma will require only a thoracostomy tube in the management of their injuries. Findings of hemodynamic lability or respiratory distress should cue the treating physician to the potential need for a chest tube. Imaging studies are not necessary in this setting. Stable patients with abnormal physical exam findings (decreased breath sounds, crepitance, hyperinflation, etc.) can proceed with portable radiographic imaging prior to undergoing thoracostomy. The ideal location for a tube thoracostomy is in the mid-axillary line at approximately the level of the xiphoid (a consistent landmark which reduces the chances of placing the tube in the major fissure). While a 36 French or larger tube has been traditionally recommended, emerging data suggest a smaller tube may be as effective. For patients in extremis, however, a 36 French tube is still recommended [4, 5]. In the setting of acute trauma, a collection system with autotransfusion capability should always be considered. There is no indication to clamp a chest tube in the setting of trauma as this increases the potential for creating an iatrogenic tension pneumothorax. In the setting of a massive air leak leading to an inability to ventilate the patient, the tube may be placed to water seal en route to the operating room for definitive pulmonary repair.
If not addressed in the field, large-bore intravenous access should rapidly be obtained above the diaphragm in patients with thoracic trauma. If appropriate peripheral access cannot be obtained, there are many commercially available rapid infusion devices for placement into the subclavian, internal jugular, or femoral veins. Alternatively, interosseous access can rapidly be obtained in the humeral heads and/or tibias presuming the accessed extremities are uninjured. Patients with penetrating injuries are at increased risk for requiring a massive transfusion (MT, >10 units of blood in the first 24 h). Numerous clinical and laboratory predictors of MT exist and have been studied. One such model, referred to as the “ABC Score,” examined non-weighted clinical parameters to determine the risk [6]. Patients with two of the following—penetrating mechanism, positive focused assessment sonography for trauma (FAST), initial SBP ≤ 90 mmHg, and arrival HR ≥ 120 bpm—are at significant increased risk for MT. As such, institutions treating unstable patients with penetrating thoracic wounds should have an MT protocol, which can be rapidly initiated.
Increased data have been reported targeting a lower systolic blood pressure in the resuscitation of patients sustaining penetrating trauma until vascular control is obtained. Following early preliminary work by Mattox, the landmark study by Bickell found patients (penetrating only) undergoing delayed resuscitation to have a survival benefit over those receiving a traditional resuscitation [7]. A large multicenter study is currently enrolling to examine if these findings are applicable to patients presenting to institutions with varying pre-hospital transport times and are inclusive of blunt injured patients.
Resuscitative thoracotomy (RT), originally described for medical cardiac arrest in the nineteenth century, gained acceptance in the trauma population soon after. The specific indications, however, have evolved since its first description and are still disputed today. There are those groups advocating a more restrictive approach and those purporting a liberal approach. Guidelines published by the American College of Surgery-Committee on Trauma (ACS-COT) discourage performing an RT on victims of blunt trauma presenting with cardiac arrest and sparingly in those that lose vital signs on presentation to the trauma center. They report the excessively low survival and low likelihood of neurological recovery. RT should be restricted to victims of penetrating trauma and in patients with witnessed physiologic parameters (vital signs, telemetric activity, pupillary reflexes, etc.) arriving to the trauma center within 15 min of EMS arrival [8]. This recommendation is supported in a recent retrospective study by Mollberg where after almost a decade of RTs at one institution, a strict adherence to the ACS-COT guidelines would account for all potential survivors while, at the same time, avoiding inappropriate resource utilization and operative hazards to healthcare providers [9]. The Western Trauma Association (WTA) has recently published guidelines promoting a more liberal approach to RT [10]. Their review, however, lacks appropriate evidence and is likely applicable to only a few trauma centers worldwide. Future evolution regarding RT is the concept of intravascular aortic occlusion. This is accomplished by inserting an inflatable balloon in the descending aorta through the femoral artery. Comparative data between this technique and the standard RT are currently time lacking.
11.6 Imaging
As in the case of all injured patients, the need for and choice of imaging studies will be dictated by the pattern of injury and clinical status. Patients presenting in extremis require few, if any, radiographs prior to proceeding to the operating room. Stable patients, conversely, may require more advanced imaging modalities depending on their injury pattern to further characterize their injuries prior to intervening.
In general, an AP chest film should be obtained sometime during the primary survey or soon thereafter. During the initial, primary survey, symptomatic, life-threatening airway and breathing problems can be addressed without imaging. Concerns for symptomatic and tension pneumothoraces should be treated without delay. The chest film is regarded as a part of the circulatory assessment in order to evaluate for thoracic hemorrhage. Hypotensive patients without signs of obstructive shock need to be evaluated for cavitary hemorrhage and a chest film is part of this assessment.
If not obtained during the primary survey, an x-ray of the chest should be performed as a resuscitative adjunct while the patient is undergoing a thorough secondary evaluation. Patients presenting with penetrating chest injuries should have radiographic markers placed over wound sites prior to imaging. If the clinical situation allows, a semierect film with the patient in the seated position is preferable. The XR is evaluated for tracheal position, parenchymal disease, hemopneumothorax, and mediastinal widening (Fig. 11.4a–c). Tube thoracostomy should be considered for all hemopneumothoraces seen on plain radiograph. Consideration should be given to connecting the chest tube to a collection system with autotransfusion capabilities. Stable patients with low-velocity penetrating injuries (i.e., stab wounds) presenting with normal radiographs may be safely discharged if a repeat film in 4–6 h fails to show the development of a pneumothorax [11]. Emerging data suggest patients may be safely discharged even earlier than the accepted 4–6 h [12]. Ultrasound has gained popularity for evaluation of blunt abdominal trauma and as such is being used with increasing frequency for the evaluation of thoracic injuries. Although operator dependent, the Focused Abdominal Sonogram for Trauma (FAST) exam carries a high specificity for the presence intraperitoneal fluid [13]. Part of the FAST exam includes a four-chamber subxiphoid view of the heart to evaluate for pericardial fluid (Fig. 11.5). In studies of patients with penetrating thoracic injuries, sensitivity and specificity have been noted as high as 97 % for cardiac injury [14]. The presence of a left hemo-/pneumothorax may reduce accuracy of ultrasound and further diagnostic workup may be necessary in this setting. Transesophageal echocardiography may have a role in diagnosing cardiac and aortic injuries. This modality, however, is more invasive and operator dependent than traditional transthoracic echocardiography.
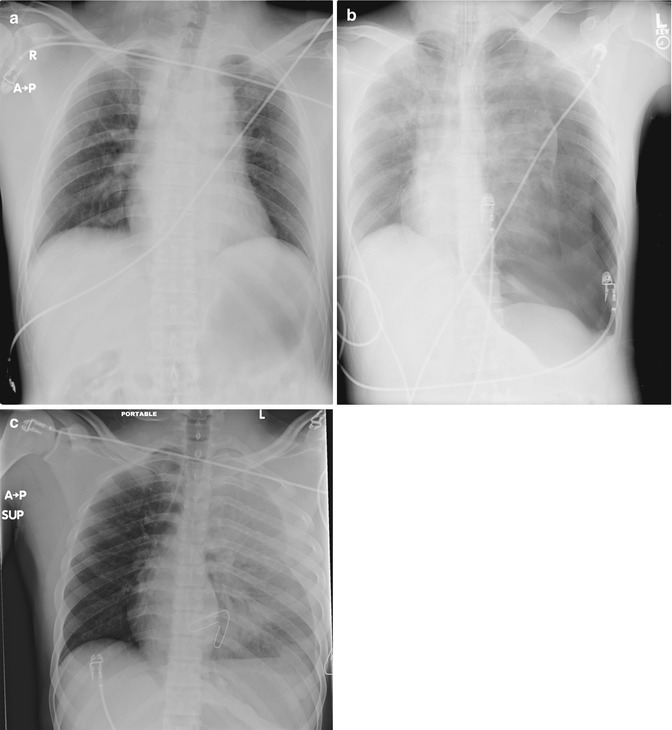
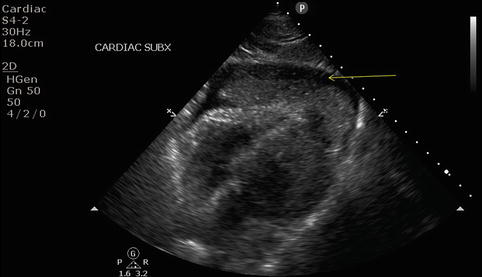

Fig. 11.4
(a) Widened mediastinum. (b) Tension pneumothorax. (c) Massive hemothorax

Fig. 11.5
FAST view of pericardial window
CT angiography has virtually replaced traditional aortography with regard to blunt chest trauma. This was evidenced in a comparison study between two similar AAST multicenter prospective studies separated by a decade (1997 and 2007) looking at blunt aortic injury [15]. The 2007 study noted a near-complete elimination of conventional angiography and TEE, with nearly all patients undergoing CT angiography [15]. Although limited prospective studies exist, stable patients presenting with transmediastinal gunshot wounds may be safely evaluated with axial imaging following appropriate primary and secondary surveys. The results of such an imaging study will determine the need for further diagnostic workup or operative interventions needed. The benefit of CTA is in its ability to delineate missile tract and vascular injury. Missile tracts remote from worrisome structures will help to limit further imaging studies. If concern for injury based on trajectory remains, further workup (e.g., endoscopy or fluoroscopy for esophageal evaluation) is warranted. Stable patients with periclavicular injuries should also have a CT angiogram to assess for vascular injury. Management of specific vascular injuries is addressed elsewhere in this text.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


