81 Idiopathic ventricular tachycardias (VTs) typically occur in the absence of structural heart disease and can originate from multiple anatomic regions, including the left and right ventricular outflow tracts (OTs), the left fascicular system, the mitral and tricuspid annuli, and the papillary muscles, or they can develop perivascularly in the epicardial space. More than half of all idiopathic VTs originate from the OTs, and of these, approximately 80% originate from the right ventricular outflow tract (RVOT).1 The remainder originate from the left ventricular outflow tract (LVOT), which is located immediately posterior to the RVOT and includes the aortic sinuses of Valsalva, the ventricular myocardium just inferior to the aortic valve, the aortomitral continuity (AMC), the superior basal septum (ventricular summit), and the epicardial OT surface.2–7 OT arrhythmias can present as isolated premature ventricular complexes (PVCs), as nonsustained VTs, or as sustained VTs.8 Symptoms of OT VTs vary widely and may include palpitations, presyncope, and syncope. Since the time that they were initially described, advances in our understanding of the mechanism and pathophysiology of OT VTs have been significant. This chapter reviews the anatomy, epidemiology, mechanisms, diagnosis, prognosis, and management of OT PVCs and VTs. The RVOT is generally divided into rightward (free wall), anterior, leftward (septal), and posterior portions (Figure 81-1).9 The RVOT courses anterior and leftward of the LVOT with the distal RVOT and pulmonic valve located leftward of the distal LVOT and aortic valve, respectively. The pulmonary valve is superior to the aortic valve, such that the posteroseptal RVOT is immediately anterior and adjacent to the aortic valve, specifically the right and left coronary cusps (RCCs and LCCs).9 Its inferior and most rightward portion is continuous with the tricuspid annulus and the interventricular septum, where the bundle of His and the proximal right bundle branch are located, whereas its most superior and leftward portion is above the anterior pulmonary valve leaflet (Figure 81-2). Although the RVOT is muscular throughout, it is relatively thin in the rightward, anterior, and subpulmonary valve portions, and it is thicker in the proximal posterior portion, where it is directly opposite the LVOT and the anterior interventricular septum. Figure 81-1 Superior View of the Human Heart With the Atria, Pulmonary Artery, and Aorta Cut Away Figure 81-2 Anterior View of the Human Heart With the Free Wall of the Right Ventricle Cut Open The left main coronary artery arises from the LCC, adjacent and immediately posterior to the RVOT below the pulmonary valve; therefore the potential for left main arterial damage exists during ablation both in the LCC and in the septal and posterior regions of the RVOT (see Figure 81-1). The left main coronary artery courses laterally relative to the RVOT as it branches into the left circumflex (LCx) and left anterior descending (LAD) arteries. Because the RVOT courses leftward, the right coronary artery (RCA), arising from the RCC, is closest to the proximal RVOT near the tricuspid annulus. The ventricular myocardium extends beyond the semilunar valves into the proximal pulmonary artery and the aortic valve cusps,10 and can be found both between and within the cusps.11 Some cases of myocardial sleeves in the region of the coronary artery have also been reported.12,13 Because the pulmonary valve is superior and leftward of the aortic valve, the RVOT beneath the pulmonary valve is at the same level as the aortic cusps. The RCC and the junction between the LCC and the RCC lie immediately adjacent to the posterior RVOT, beneath the pulmonary valve. Myocardial sleeves are found most often in the RCC and less commonly in the LCC.10 The NCC lies posterior and immediately adjacent to the interatrial septum; therefore its myocardial sleeves may be a site of origin for atrial tachycardias but not for idiopathic VTs.14 Approximately 9% of idiopathic VTs originate epicardially, most often near coronary venous sites: the great cardiac vein (GCV), the anterior interventricular vein (AIV), and the middle cardiac vein (MCV).15 Immediately distal (in the retrograde direction to blood flow) to the coronary sinus (CS) os, the CS gives rise to the MCV, which courses in the posterior interventricular sulcus. The CS courses in the inferior aspect of the atrioventricular groove parallel to the mitral valve annulus, and ends at the valve of Vieussens in the region of obtuse marginal arteries. Beyond the valve of Vieussens, the vein continues as the GCV, coursing epicardially directly overlying the lateral mitral annulus. The junction of the AIV and the GCV is immediately lateral to the LCC. Proximal to distal, the AIV is adjacent to the posterolateral subvalvar RVOT, or the epicardial lateral RVOT, and the anterior epicardial space. Transpericardial mapping of the origin of epicardial idiopathic VTs has confirmed their propensity for origin near these perivascular structures.15 OT VTs may also originate from the region of the LV summit, the most superior LV epicardial region at the top of the interventricular septum. The LV summit lies between the LAD and LCx arteries, near the junction of the GCV and the AIV, and superior to the aortic valve cusps.16 One series reported that 12% of idiopathic LV VTs originate from the LV summit.16 The superior-most region of the LV summit is close to the LAD and LCx arteries, along with prominent overlying epicardial fat, thus is inaccessible to epicardial catheter ablation; the lateral region is accessible via the GCV. OT VTs have accounted for approximately 10% of all patients referred for evaluation of VT.17 OT VTs may present from the spectrum of premature ventricular complexes, nonsustained VT, and sustained monomorphic VT.8 OT VT typically occurs in young to middle-aged patients, with mean age at presentation lower than that of patients with VT secondary to structural heart disease.18,19 Although some have noted a higher incidence in women,2 others have reported no gender predominance.8 Palpitations, chest pain, dyspnea, and light-headedness during episodes are common, and rarely syncope can occur; some patients may be entirely asymptomatic.18 Episodes are often triggered by caffeine, emotional stress, or exercise, typically during the recovery period after exercise.18,20,21 In women, hormonal flux (e.g., premenstrual, perimenopausal) may be a particular trigger.21 Circadian variation with morning and late afternoon peaks of occurrence of outflow tract PVCs has also been reported.22 These suggest the importance of sympathetic activity and circulating catecholamines in the mechanism of OT VTs. The mechanism of OT VTs is triggered activity due to cyclic adenosine monophosphate (cAMP)-mediated delayed afterdepolarizations (DADs).1,23 Increased cAMP can occur with beta-adrenergic receptor stimulation (e.g., during exercise), resulting in increased intracellular calcium and DADs, triggering OT VT. Consistent with this mechanism, in the electrophysiology (EP) laboratory, OT PVCs and VTs typically are induced by burst atrial or ventricular pacing rather than by programmed stimulation (which would be more characteristic of monomorphic VT due to reentrant mechanism) and are facilitated by isoproterenol or epinephrine infusion.18,24 These VTs cease with adenosine, verapamil, and β-blockers, all of which interfere with the cAMP-mediated calcium flux.25,26 Recognition and localization of OT arrhythmias are ideally suited to the 12-lead electrocardiogram (ECG)18 because they originate from a focal source and usually occur in a structurally normal heart. The classic ECG pattern consists of left bundle branch block morphology in V1 with an inferior axis (Figure 81-3). This occurs because the PVC/VT originates from the superior-most aspect of the ventricle, just at or below the level of the semilunar valves. Recognizing the typical ECG pattern is important for establishing the correct diagnosis and for discussing the prognosis with the patient. More detailed ECG localization is helpful when treatment options and the risks of catheter ablation are discussed, and when a starting point is identified from which more detailed intracardiac mapping can be performed in the EP laboratory. Figure 81-3 12-Lead ECG of a Patient With a Typical RVOT PVC The most common origin of OT VT is the septal aspect of the RVOT, just inferior to the pulmonic valve (see Figure 81-2).27 Because the PVC/VT exits from the superior-most region of the right ventricle, the inferior leads in stereotypical ECG morphology are markedly positive and narrow (see Figure 81-3). Leads aVR and aVL are markedly negative with a QS pattern. All RVOT PVCs/VT have left bundle branch block morphology in lead V1 (rS or QS; see Figure 81-3). The precordial lead transition from net negative to net positive (from r<S to R>s) is critical for distinguishing RVOT from LVOT origin. The transition for PVCs of RV origin typically occurs at or later than lead V3. Lead I is typically of low amplitude and is useful for differentiating posteroseptal (positive) from midseptal (multiphasic) or anteroseptal (negative) origin (Figure 81-4). The more leftward location of the anterior RVOT compared with the posterior RVOT accounts for the differences in lead I polarity (see Figures 81-1 and 81-4). The most common site of PVC origin is the anteroseptal or midseptal region.27 Less than 10% of RVOT PVCs originate from the free wall.28 For free-wall RVOT PVCs, the QRS remains inferiorly directed but is wider and notched compared with those of septal origin (see Figure 81-4). In the study by Dixit and colleagues,29 no QRS width cutoff clearly separated free-wall from septal origin; however, relative QRS widening with free-wall compared with septal origin was clearly noted within each patient. Because the RV free wall is more anteriorly located, the precordial transition is seen later than that of VT with septal origin, nearly always at or later than lead V4 (see Figure 81-4). Similar to septal PVCs, lead I is useful for differentiating anterior (negative) from posterior (positive) free-wall origin. Because a free-wall origin is very uncommon in patients with a structurally normal heart, the presence of PVCs of free-wall morphology should elicit a more thorough search for underlying structural heart disease, in particular arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). Figure 81-4 Typical 12-Lead ECG Morphologies of the OT VTs, All from Patients With the Origin Confirmed With Intracardiac Mapping and Ablation PVCs may also originate lower in the OT, further beneath the pulmonic valve. As the origin becomes more inferior, a series of changes to the ECG occurs: The inferior leads become less positive (first lead III, then lead aVF, and finally lead II), lead aVL becomes less negative, and lead I becomes more positive (as the origin becomes more posterior toward the tricuspid valve; Figure 81-2). An ECG morphology that is slightly inferiorly directed (positive in II and aVF and biphasic or positive in III), flat, or w-shaped in lead aVL and is more positive in lead I than in the typical posteroseptal PVC is typical for a PVC that originates from the RV inflow tract at the level of the His bundle30 (see Figure 81-4). These para-Hisian PVCs are less common than OT PVCs but do occur in structurally normal hearts. The origin may be just superior or free-wall to the His bundle region, and careful mapping is critical to avoid heart block during ablation. Even more infrequently, PVCs may originate superior to the pulmonic valve from myocardial fibers that extend into or just above the pulmonary valve.31 Few ECG features are helpful in distinguishing pulmonary artery (PA) from RVOT PVCs, other than a markedly inferior axis, earlier precordial transition (>R/S ratio in lead V2), and the presence of a small atrial and ventricular potential on the mapping catheter at the site of successful ablation.32 Pulmonary artery origin can be confirmed with right ventriculography or intracardiac echocardiography (ICE). Recognition that OT PVCs can also originate from the LVOT is increasing.33 The most common origin is from myocardial fibers within or adjacent to the aortic root,34,35 but PVCs may also originate from the left ventricular (LV) myocardium just below the aortic valve, from the LV summit at the top of the interventricular septum, from the region of the AMC, or from the LV outflow epicardium.36 LVOT PVCs also have a left bundle branch block pattern in lead V1 (usually rS) with an inferior axis. Because the aortic root is immediately posterior to the RVOT (see Figure 81-1), LVOT PVCs have an earlier precordial transition, typically at or before lead V3.11,37 Further analysis can be performed to identify the origin within the aortic root. PVCs from the LCC region typically have an rS in lead I and an earlier precordial transition (lead V1 or V2) than those from the RCC (see Figure 81-4). PVCs from the RCC typically are positive and notched in lead I, with a precordial transition at lead V2 or V3.38 The region at the junction of the RCC and LCC is a common site of aortic root PVC origin and has a signature “notch” or qrS pattern in lead V1 or V239,40 (see Figure 81-4). As was already mentioned, PVCs with a precordial transition before lead V3 typically originate in the LVOT, and those with a transition after V3 typically originate in the RVOT. PVCs with a transition at lead V3 are therefore challenging to localize.41 In addition, cardiac rotation or precordial lead misplacement can confound most ECG localization algorithms. One study found that comparing the precordial transition of the PVC with that in sinus rhythm can be helpful in localizing PVCs with a V3 transition. If the PVC transition occurs after the sinus rhythm transition, the origin is RVOT with 93% specificity.42 Other algorithms are available for distinguishing RVOT from LVOT arrhythmias43,44 (Table 81-1, Distinguishing ECG Features of LVOT vs. RVOT PVCs).
Outflow Tract Ventricular Tachyarrhythmias
Mechanisms, Clinical Features, and Management
Anatomy
RVOT
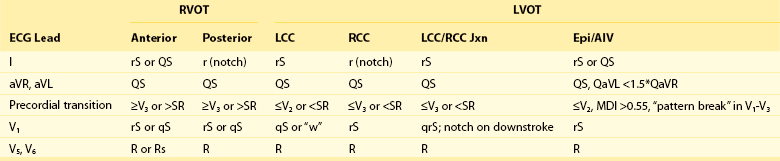
The heart is oriented with the anterior (A) aspect of the chest at the top, posterior (P) at the bottom, and right (R) and left (L) sides in the appropriate orientation. The location of the ECG leads on the chest is shown. The most anterior and superior part of the ventricles is the right ventricular free wall just below the pulmonic valve, which is separated into anterior (AF), mid (MF), and posterior (PF) free-wall regions. Just posterior to the free wall is the septal aspect of the RVOT, separated into anterior (AS), mid (MS), and posterior (PS) septal regions. Immediately posterior and slightly inferior to the RVOT septum lies the aortic root. The right coronary cusp (R) lies immediately posterior to the PS RVOT, and the left coronary cusp (L) lies posterior to the AS RVOT. The noncoronary cusp (N) overlies the atrial septum and therefore is a useful location for mapping atrial tachycardia but not OT VTs. Posterior to the aortic root lies the mitral annulus (MA), below the left atrium (LA).
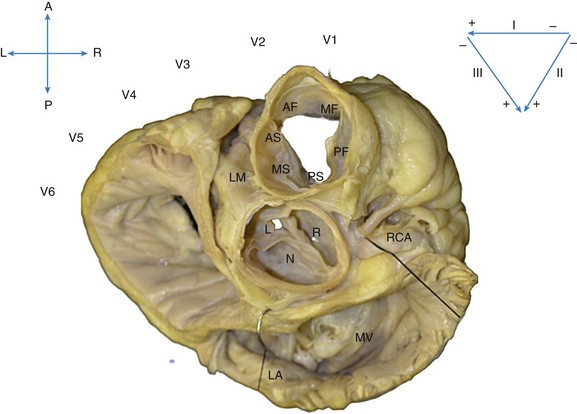
This view shows the anteroseptal (AS), midseptal (MS), and posteroseptal (PS) aspects of the RVOT just below the pulmonic valve (PV). Immediately posterior to the RVOT lies the aortic root (Ao). Inferior and posterior to the RVOT lies the para-Hisian region (His), a less common site of origin of idiopathic PVCs. This region at the superior aspect of the tricuspid valve has been termed the tricuspid “inflow tract.”
Relationship to Coronary Arteries
Aortic and Pulmonary Valve Cusps
Epicardial and Perivascular Sites
Left Ventricular Summit
Epidemiology
Symptoms
Triggers
Mechanism
Diagnosis
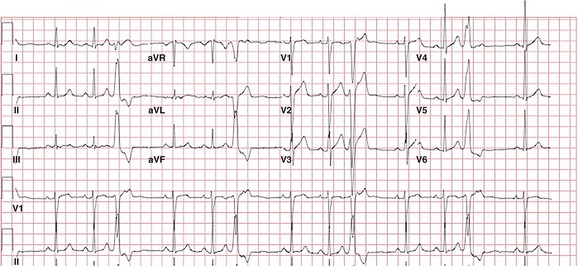
The PVC has a left bundle branch block pattern in V1 with an rS—a transition at lead V4 that is typical of RVOT PVCs. The PVC has a marked inferior axis and is multiphasic in lead I, suggesting a midseptal origin. Although notching in the inferior leads is more commonly seen in PVCs of free-wall origin, it can also be seen in PVCs of septal origin.
RVOT

From right to left, para-Hisian PVCs are not really located in the outflow tract but do have a left bundle inferior axis; the hallmark is the isoelectrical or “w” pattern in lead aVL and the more positive R wave in lead I. Next, an anterior free-wall (AF) PVC is shown, with the characteristic lower amplitude and notched inferior leads and a late precordial transition. The more typical septal origin PVCs are shown next; lead I distinguishes anteroseptal (AS, negative) from midseptal (MS, multiphasic) or posteroseptal (PS, positive) origin. The coronary cusp PVCs all have left bundle branch block (LBBB) morphology in V1 with an earlier precordial transition. More posteriorly located is the region of aortomitral continuity (AMC), which has an inferior axis and a signature qR pattern in lead V1. See text for a more detailed discussion.
LVOT
Outflow Tract Ventricular Tachyarrhythmias: Mechanisms, Clinical Features, and Management