The goal of this study was to assess outcomes of patients who underwent implantation of left ventricular assist devices (LVADs) at nontransplantation mechanical circulatory support centers. As the availability of LVADs for advanced heart failure has expanded to nontransplantation mechanical circulatory support centers, concerns have been expressed about maintaining good outcomes. Demographics and outcomes were evaluated in 276 patients with advanced heart failure who underwent implantation of LVADs as bridge to transplantation or destination therapy at 27 open-heart centers. Baseline characteristics, operative mortality, length of stay, readmission rate, adverse events, quality of life, and survival were analyzed. The overall 30-day mortality was 3% (8 of 276), and survival rates at 6, 12, and 24 months, respectively, were 92 ± 2%, 88 ± 3%, and 84 ± 4% for the bridge-to-transplantation group and 81 ± 3%, 70 ± 5%, and 63 ± 6% for the destination therapy group, comparable with results published by the national Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). The median length of stay for all patients was 21 days. Bleeding was the most frequent adverse event. Stroke occurred in 4% (bridge to transplantation) and 6% (destination therapy) of patients. Quality-of-life measures and 6-minute walk distances showed sustained improvements throughout support. In conclusion, outcomes with LVAD support at open-heart centers are acceptable and comparable with results from the INTERMACS registry. With appropriate teams, training, center commitment, and certification, LVAD therapy is being disseminated in a responsible way to open-heart centers.
Treatment of advanced-stage heart failure with left ventricular assist device (LVAD) support provides quality-of-life and survival benefit in most recipients. The development of LVAD technology has been closely associated with heart transplantation, while the greatest potential for expanded use is for destination therapy (DT) in nontransplantation candidates. The use of LVADs beyond heart transplantation centers has been somewhat controversial because of concerns about adequate training, clinical experience, and the availability of necessary resources. In 2009, after commercial approval of the HeartMate II LVAD (Thoratec Corporation, Pleasanton, California) for bridge to transplantation (BTT), nontransplantation mechanical circulatory support (MCS) centers began implanting this device with the support of partnering MCS-transplantation centers. Today, LVAD implantation for BTT and DT is performed regularly at MCS centers. This report provides outcome data for patients who underwent LVAD implantation as BTT and DT at nontransplantation MCS centers.
Methods
This retrospective study was conducted using data from 276 patients who underwent implantation of the HeartMate II LVAD at 27 MCS centers as BTT and DT. Patient data are presented by indication, BTT (n = 100) or DT (n = 176), as identified by the implanting center. Implantation of the HeartMate II LVAD at the MCS centers began in April 2009, after commercial approval of the device as BTT. Before starting LVAD implantation at the MCS centers, agreements were established with partnering MCS-transplantation centers to ensure that heart transplantation candidates were evaluated and listed according to the guidelines of the United Network for Organ Sharing. The partnering MCS-transplantation centers provided workup and care protocols specific to their programs and served as resources for information on case management as needed.
All patients were enrolled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) and gave informed consent that was approved by the institutional review board at each of the participating centers. The time frame for the data used in this study extends from the date of the first HeartMate II implantation as BTT at an MCS center (April 2009) to September 30, 2012. The first implantation for the DT indication occurred in January 2012. Each participating center entered data into the registry according to instructions in the INTERMACS manual of operations. Data obtained from INTERMACS for this study were the subset of patients who underwent implantation at the MCS centers.
Baseline characteristics, operative mortality, major adverse event rates, length of stay in the hospital, rehospitalization rate, physical status, quality-of-life measures, and survival to 2 years were analyzed. Data for 30-day, 6-month, and 12-month survival that were grouped by INTERMACS profiles (profiles 1, 2, 3, and 4 to 7) were assessed for differences based on severity of illness. Reported adverse events are defined by the INTERMACS protocol. Physical status evaluated with the 6-minute walk test and quality-of-life measures evaluated with the EuroQol EQ-5D visual analog scale and total quality-of-life scores at baseline, 3, 6, and 12 months were analyzed. The total quality-of-life score is a composite score evaluating anxieties, mobility, pain, and self-care.
Continuous data are reported as mean ± SD, and discrete variables are reported as percentages. Survival analysis was performed using the Kaplan-Meier method with censoring when LVAD support was stopped for heart transplantation or when recovery enabled device removal. Adverse events are given as the total number of events and the event rate (number of events divided by total patient-years). Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Data are presented in 2 groups according to indication for LVAD support (BTT or DT), but statistical comparisons between the groups were not performed.
Results
The baseline characteristics for patients who underwent implantation of the HeartMate II as BTT (n = 100), as DT (n = 176), and the 2 groups combined (n = 276) are listed in Table 1 . The 2 groups were composed predominantly of men, and the age distribution (ranges defined by INTERMACS) was consistent with the indication for support (i.e., 35% of patients were >70 years of age in the DT group, and only 6% were >70 years of age in the BTT group). Most BTT patients (67%) were 50 to 69 years of age, and those in the DT group were predominately >60 years of age (72%). The severity of illness defined on the basis of INTERMACS profile was similar for the 2 groups. Patients with critical cardiogenic shock (INTERMACS profile 1) represented the smallest proportion in both groups. Hemodynamic and laboratory parameters for the 2 groups were representative of advanced-stage heart failure.
| Variable | Bridge-to-Transplant (n = 100) | Destination Therapy (n = 176) | All (N = 276) |
|---|---|---|---|
| Males | 81 (81%) | 145 (82%) | 226 (82%) |
| Age (Years) | |||
| ≤59 | 62 (62%) | 49 (28%) | 111 (40%) |
| 60–69 | 32 (32%) | 65 (37%) | 97 (35%) |
| ≥70 | 6 (6%) | 62 (35%) | 68 (25%) |
| Heart Failure Etiology | |||
| Ischemic cardiomyopathy | 43 (43%) | 103 (59%) | 146 (53%) |
| Idiopathic cardiomyopathy | 34 (34%) | 48 (27%) | 82 (30%) |
| Other etiology | 24 (24%) | 25 (14%) | 49 (18%) |
| INTERMACS Profile | |||
| 1: Critical cardiogenic shock | 7 (7%) | 17 (10%) | 24 (9%) |
| 2: Progressive decline | 32 (32%) | 52 (30%) | 84 (30%) |
| 3: Stable but inotrope dependent | 44 (44%) | 72 (41%) | 116 (42%) |
| 4–7: Non-inotrope dependent | 17 (17%) | 35 (20%) | 52 (19%) |
| Hemodynamics | |||
| Heart rate (bpm) | 85.8 ±17.7 | 84.9 ±15.0 | 85.2 ±16.1 |
| Mean blood pressure (mmHg) | 78.7 ±10.6 | 76.7 ±11.1 | 77.4 ±11.0 |
| Pulmonary wedge pressure (mmHg) | 22.9 ±10.4 | 22.9 ±8.1 | 22.9 ±9.0 |
| Right atrial pressure (mmHg) | 13.2 ±8.4 | 13.8 ±7.7 | 13.6 ±7.9 |
| Cardiac index (L/min/m 2 ) | 2.14 ±0.63 | 2.12 ±0.67 | 2.12 ±0.65 |
| Laboratory Data | |||
| Blood urea nitrogen (mg/L) | 23.8 ±13.4 | 29.4 ±15.0 | 27.4 ±14.6 |
| Creatinine (mg/dL) | 1.25 ±0.47 | 1.46 ±0.72 | 1.39 ±0.65 |
| Total bilirubin (mg/dL) | 1.34 ±1.14 | 1.40 ±1.61 | 1.38 ±1.46 |
| Serum glutamic oxaloacetic transaminase (u/L) | 39.6 ±30.5 | 49.0 ±96.3 | 45.6 ±79.0 |
| Serum glutamic pyruvic transaminase (u/L) | 45.8 ±49.8 | 50.5 ±87.3 | 48.8 ±75.7 |
| International normalized ratio | 1.36 ±0.46 | 1.29 ±0.23 | 1.31 ±0.33 |
The average duration of support for all MCS center patients (n = 276) was 8.3 ± 7.2 months (median 6.2, range 0 to 36.4) and was 9.6 ± 7.6 months for BTT (maximum 36.4) and 7.6 ± 6.8 months for DT (maximum 31.2). Shorter follow-up of DT patients occurred because centers only more recently started DT programs. The cumulative follow-up duration was 81.0 patient-years for BTT and 109.8 patient-years for DT.
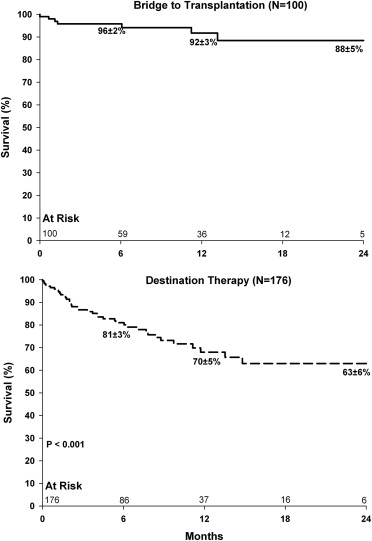
At the time of the analysis cutoff date (December 2012), 20 of the BTT patients (20%) and 7 of the DT patients (4%) had undergone heart transplantation. Death during support occurred in 8 of 100 BTT patients (8%) and in 40 of 176 DT patients (23%). The number of patients remaining on LVAD support was 195 of 276 (71%): 68 of 100 BTT patients (68%) and 127 of 176 DT patients (72%). One patient had the device explanted after myocardial recovery, 4 patients underwent device exchange because of thrombosis, and 2 devices were exchanged for unspecified device malfunctions. The overall 30-day mortality was 3% (8 of 276), with 2 deaths in the BTT group that were INTERMACS profile 2 and profile 3, respectively, and 6 deaths in the DT group that were INTERMACS profile 1 (n = 3) or profile 2 (n = 3). The survival rates by INTERMACS profile are given in Table 2 . The Kaplan-Meier survival rates ( Figure 1 ) at 6, 12, and 24 months, respectively, for the BTT group were 92 ± 2%, 88 ± 3%, and 84 ± 4%; for the DT group, the respective survival rates were 81 ± 3%, 70 ± 5%, and 63 ± 6%.
| Profile | Bridge to Transplant | Destination Therapy | ||
|---|---|---|---|---|
| 6-Month (n = 59) | 12-Month (n = 36) | 6-Month (n = 86) | 12-Month (n = 37) | |
| 1 | 100%(2) | 100% (1) | 63.4 ±12.0% (7) | NA |
| 2 | 96.9 ±3.1% (20) | 91.8 ±5.8% (10) | 82.4 ±5.7% (28) | 64.3 ±8.5% (16) |
| 3 | 92.7 ±4.1% (28) | 88.0 ±6.0% (19) | 81.5 ±5.1% (37) | 76.4 ±5.9% (13) |
| 4–7 | 100% (9) | 100% (6) | 87.9 ±6.7% (14) | 72.6 ±11.5% (8) |
The 276 patients underwent implantation of the HeartMate II at 27 MCS centers with an average of 10.3 ± 11.7 (range 1 to 55) implantations per center ( Table 3 ). The survival rates at 1, 6, and 12 months at the centers with ≤20 implants (n = 127) were 96.8 ± 1.6%, 89.8 ± 3.0%, and 78.2 ± 5.2%, respectively, and at centers with >20 implantations, the rates were 98.0 ± 2.0%, 85.6 ± 3.1%, and 80.4 ± 3.9%, respectively (p = 0.702).
| SMCS Center | Bridge to Transplant | Destination Therapy | Total |
|---|---|---|---|
| Abington Memorial | 0 | 1 | 1 |
| Albert Einstein | 1 | 0 | 1 |
| Banner Good Samaritan | 0 | 2 | 2 |
| Hackensack University Med Ctr. | 3 | 0 | 3 |
| Lancaster General | 1 | 11 | 12 |
| Lankenau Hospital | 0 | 4 | 4 |
| Morristown Memorial Hospital | 6 | 14 | 20 |
| MultiCare Health System | 0 | 6 | 6 |
| New York University Med Ctr. | 6 | 6 | 12 |
| OSF St Francis Med Ctr. | 2 | 1 | 3 |
| Penn Presbyterian Med Ctr. | 1 | 7 | 8 |
| Piedmont Hospital | 14 | 41 | 55 |
| Providence St Vincent Med Ctr. | 2 | 9 | 11 |
| Scott & White Hospital | 4 | 8 | 12 |
| Scripps Memorial Hospital | 1 | 0 | 1 |
| Spectrum health Hospitals | 7 | 21 | 28 |
| St Mary’s Hospital | 17 | 8 | 25 |
| Stony Brook University Med Ctr. | 7 | 2 | 9 |
| The Heart Hospital Baylor Plano | 0 | 3 | 3 |
| The Christ Hospital | 11 | 5 | 16 |
| The Indiana Heart Hospital | 1 | 1 | 2 |
| The Med Ctr. of Central Georgia | 0 | 3 | 3 |
| University of Toledo | 1 | 4 | 5 |
| UC Health University Hospital | 0 | 8 | 8 |
| UC Davis Med Ctr. | 1 | 2 | 3 |
| UC San Diego Med Ctr. | 1 | 1 | 2 |
| Weill Cornell Med Ctr. | 16 | 8 | 24 |
After LVAD implantation, the median length of stay in the hospital was 21 days for all MCS center patients, and it was 6 days longer for DT patients (22.5 days) than for BTT patients (16.5 days). The percentages of patients discharged after LVAD implantation were 92% for BTT patients, 90% for DT patients, and 91% for all patients. The hospital readmission rate was 1.48 events per patient-year (1.10 for BTT, 1.77 for DT). The reasons for readmission in the DT cohort are listed in Table 4 .
| Reason | Number of readmissions |
|---|---|
| Cardiac arrhythmia | 6 |
| Bleeding | 13 |
| Hematoma | 1 |
| Hemolysis | 1 |
| Infection | 9 |
| Gastrointestinal disorder | 6 |
| Gastrointestinal bleeding | 11 |
| Planned procedure | 3 |
| Device malfunction | 1 |
| Suspected device thrombosis | 1 |
| Neurologic event | 6 |
| Psychiatric event | 1 |
| Renal dysfunction | 1 |
| Wound dehiscence | 2 |
| Other not specified | 24 |
| Multiple events | 22 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


