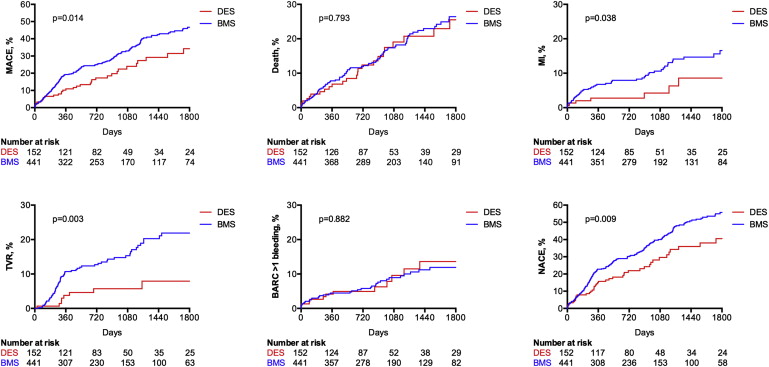
Limited data are available on long-term efficacy and safety of drug-eluting stents (DES) in elderly patients who underwent PCI. A total of 635 consecutive patients aged ≥75 years who underwent PCI were enrolled at 2 European centers. Of these, 170 patients received at least 1 DES, whereas 465 patients received bare metal stent (BMS) only. Primary end point was the incidence of net adverse clinical events (NACE), defined as the occurrence of ischemic events or bleeding events, and was compared at a median follow-up of 31.2 months. Clinical follow-up information was available in 593 patients (93.4%). The duration of dual antiplatelet therapy was 12.3 ± 5.1 months in the DES group and 3.8 ± 7.4 months in the BMS group. The Kaplan-Meier estimate of NACE at 5 years was significantly lower in DES-treated patients (40.5%) than in BMS-treated patients (55.7%; p = 0.009). This benefit was driven by a significant reduction in myocardial infarction (8.6% vs 16.6%; p = 0.038) and target vessel revascularization rates (7.9% vs 21.9%; p = 0.003) in the DES group, with no significant increase in the incidence of bleeding events (13.8% vs 12.2%; p = 0.882). These results were confirmed at propensity score–adjusted Cox proportional hazard analysis. In conclusion, in patients ≥75 years, the use of DES compared with BMS seems to reduce myocardial infarction and repeat revascularization rates at long-term follow-up, without an increase in bleeding despite longer duration of dual antiplatelet therapy. This net clinical benefit, resulting from persistent efficacy and safety over time, may support the use of DES as a reasonable option in patients ≥75 years.
Drug-eluting stents (DES) are effective in reducing the incidence of restenosis and repeat revascularization especially in the subset of complex lesion, such as multivessel disease, small-vessel disease, long lesions, and in patients with diabetes. Older patients may benefit more from DES implantation because of their greater anatomical complexity. Although the safety of second-generation DES regarding stent thrombosis (ST) has been largely documented, concerns about the need of prolonged dual antiplatelet therapy (DAPT) in a population with high incidence of co-morbidities and high bleeding risk continue to constrain the use of DES in elderly patients. Limited evidence is available on long-term outcomes of such patients who underwent PCI with DES, especially regarding safety data on bleeding events. Aim of the present study was to assess long-term net clinical outcomes of elderly patients treated with DES compared with bare metal stent (BMS).
Methods
Consecutive elderly patients (≥75 years) who underwent elective or urgent PCI were enrolled at the Department of Cardiovascular Sciences, Campus Bio-Medico University of Rome, Rome, Italy, and at the Cardiovascular Center Aalst, Aalst, Belgium, over a 2-year period (2008 to 2010). Exclusion criteria were PCI performed without stent implantation or with both DES and BMS, left ventricular ejection fraction <30%, chronic kidney disease with an estimated glomerular filtration rate <15 ml/min/1.73 m 2 , high bleeding risk (active internal bleeding, history of hemorrhagic stroke, intracranial neoplasm, arteriovenous malformation or aneurysm, ischemic stroke in the previous 3 months), and coronary artery bypass surgery in the previous 3 months.
The type of stent implanted (BMS or DES) was left at the physician’s discretion. Pre-PCI antiplatelet treatment consisted of aspirin (80 to 100 mg/d) and clopidogrel 600-mg loading dose before the procedure or 75 mg/d for at least 5 days. Periprocedural use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. Procedural anticoagulation consisted of unfractionated heparin administrated to achieve an activated clotting time of 250 to 300 seconds. After PCI, patients receiving BMS were prescribed clopidogrel 75 mg for at least 4 weeks, whereas in those with non–ST-elevation acute coronary syndrome (ACS) or who underwent DES implantation clopidogrel 75 mg was indicated for 12 months. Low-dose aspirin was continued indefinitely. This study complied with the Declaration of Helsinki and was approved by the local ethics committees, with all patients giving written informed consent.
Clinical follow-up information was obtained by office visit, telephone interview, or chart review. All events were classified and adjudicated by a physician not involved in the follow-up process. Primary end point of our study was the incidence of net adverse clinical events (NACE), defined as the occurrence of ischemic or bleeding events at follow-up. Secondary end point was the incidence of the single components of the primary end point. Ischemic events were defined as death, nonfatal myocardial infarction (MI), and target-vessel revascularization (TVR). Nonfatal MI included spontaneous events, defined as detection of an increase and/or decrease of cardiac biomarker values with at least 1 value more than the ninety-ninth percentile upper reference limit. In addition, patients had to present one of the following features: symptoms of ischemia, new, or presumed new significant ST-segment T-wave changes or new left bundle branch block; development of pathological Q waves in the ECG; or imaging evidence of new loss of viable myocardium, new regional wall motion abnormality, identification of an intracoronary thrombus by angiography, or autopsy. TVR was clinically driven and included bypass surgery or repeat PCI of the target vessels. Definite and probable ST were monitored and defined according to the Academic Research Consortium definition. Bleeding events were defined according to the Bleeding Academic Research Consortium (BARC) criteria. BARC class >1 bleeding was considered for this analysis.
Categorical variables are reported as frequencies and percentages. Continuous variables are reported as mean ± SD. Comparisons between continuous variables were performed using the Student’s t test or Mann-Whitney U test, as appropriate. Comparisons between categorical variables were evaluated using the Fisher’s exact test or the Pearson’s chi-square test, as appropriate. A propensity score was built with a nonparsimonious method to account for potential differences in treatment allocation and then entered into a logistic regression model. In particular, all variables listed in Tables 1 and 2 , with the exception of stent type, were incorporated into the model, and the score was then used in proportional hazard analyses as a covariate. Kaplan-Meier method was used to estimate survival curves and the log-rank test to evaluate differences between groups. Cox proportional hazard analysis was used to estimate the association between stent type and long-term clinical outcomes, after adjusting for propensity score and, in a separate model, for the enrolling center. Statistical analysis was performed using Stata/IC, version 11.0 (STATA Corp., College Station, Texas), and p values <0.05 (2 tailed) were considered significant.
| Variable | Overall (n=635) | DES (n=170) | BMS (n=465) | p value |
|---|---|---|---|---|
| Age (years) | 79.9±3.6 | 79.9±3.7 | 79.4±3.5 | 0.097 |
| Men | 417 (67%) | 120 (71%) | 297 (64%) | 0.114 |
| Body mass index (kg/m 2 ) | 26.9±4.2 | 27.4±4.4 | 26.6±4.1 | 0.047 |
| Systemic hypertension ∗ | 519 (82%) | 134 (79%) | 385 (83%) | 0.251 |
| Dyslipidemia † | 459 (72%) | 119 (70%) | 340 (73%) | 0.437 |
| Diabetes mellitus | 247 (39%) | 88 (52%) | 159 (34%) | <0.001 |
| Smoker | 114 (18%) | 50 (29%) | 64 (14%) | <0.001 |
| Left ventricle ejection fraction (%) | 53.3±9.1 | 54.6±8.2 | 53.0±9.3 | 0.293 |
| Prior myocardial infarction | 152 (24%) | 36 (21%) | 116 (25%) | 0.023 |
| Prior percutaneous coronary intervention | 89 (14%) | 28 (16%) | 61 (13%) | 0.302 |
| Prior coronary by-pass | 45 (24%) | 30 (29%) | 15 (18%) | 0.302 |
| Chronic kidney disease ‡ | 446 (70%) | 121 (71%) | 325 (70%) | 0.423 |
| Clinical presentation | 0.252 | |||
| Stable angina pectoris | 427 (67%) | 120 (71%) | 307 (66%) | |
| Non-ST-elevation ACS | 175 (28%) | 45 (26%) | 130 (28%) | |
| ST segment elevation myocardial infarction | 33 (5%) | 5 (3%) | 28 (6%) | |
| Diseased vessels | 2.03±1.04 | 2.08±0.92 | 2.02±1.08 | 0.540 |
∗ Systolic blood pressure: >140 mm Hg and/or diastolic blood pressure >90 mm Hg or current antihypertensive treatment.
† Total cholesterol >200 mg/dl or statin therapy.
‡ Chronic kidney disease: estimated glomerular filtration rate <60 ml/min/1.73 m 2 .
| Variable | Overall (n=635) | DES (n=170) | BMS (n=465) | p value |
|---|---|---|---|---|
| Treated coronary vessel | 0.209 | |||
| Left main | 26 (3%) | 11 (5%) | 15 (3%) | |
| Left anterior descending | 370 (46%) | 95 (47%) | 277 (45%) | |
| Left circumflex | 138 (17%) | 30 (15%) | 107 (18%) | |
| Right | 251 (32%) | 60 (30%) | 190 (32%) | |
| Saphenous vein graft | 14 (2%) | 5 (2%) | 9 (2%) | |
| Multivessel coronary angioplasty | 144 (23%) | 30 (18%) | 114 (25%) | 0.067 |
| Chronic total occlusion | 31 (5%) | 10 (6%) | 21 (5%) | 0.533 |
| Bifurcation lesion | 30 (5%) | 16 (9%) | 14 (3%) | 0.001 |
| Type of drug eluting stent | – | |||
| sirolimus | – | 13 (8%) | – | |
| paclitaxel | – | 15 (9%) | – | |
| zotarolimus | – | 54 (32%) | – | |
| everolimus | – | 88 (51%) | – | |
| Number of stents implanted | 1.76±1.24 | 1.72±1.03 | 1.78±1.30 | 0.187 |
| Total stent length (mm) | 32.47±22.59 | 31.05±19.45 | 32.99±23.64 | 0.339 |
| Stent diameter (mm) | 3.16±3.13 | 3.10±0.37 | 3.19±0.38 | 0.013 |
Results
A total of 635 patients fulfilling inclusion and exclusion criteria were recruited in this study (354 patients enrolled at Campus Bio-Medico University of Rome and 281 patients enrolled at Cardiovascular Center Aalst). Of these, 170 patients received at least 1 DES, whereas 465 patients were treated with BMS only. Main clinical and procedural features are listed in Tables 1 and 2 , respectively. Clinical presentation was stable angina in 427 patients (67%), non–ST-elevation ACS in 175 patients (28%), and ST-elevation MI in 33 patients (5%). Patients treated with DES were more likely to be diabetic, smokers, with higher body mass index, and more frequently had a history of MI than BMS-treated patients. Stent diameter was significantly lower in the DES group, but no difference was identified in total stent length and number of stent implanted. Clinical and procedural characteristics were homogeneous in patients enrolled in the 2 centers, except for diabetes mellitus, which was more frequent in patients enrolled at Campus Bio-Medico University of Rome (42.4% vs 34.5%, p = 0.049).
Clinical follow-up information was available in 593 patients (93.4%). The median follow-up duration was 29.9 months in the DES group and 33.4 in the BMS group, which were not significantly different. The duration of dual antiplatelet therapy was 12.3 ± 5.1 months in the DES group and 3.8 ± 7.4 months in the BMS group. The unadjusted Kaplan-Meier estimates of major adverse cardiac event (MACE; 34.2% vs 46.7%; p = 0.014), nonfatal MI (8.6% vs 16.6%; p = 0.038), TVR (7.9% vs 21.9%; p = 0.003), and NACE (40.5% vs 55.7%; p = 0.009) at 5 years were significantly lower in the DES group compared with the BMS group, whereas no significant difference was found in the estimates of death (25.5% vs 26.4%; p = 0.793) and BARC class >1 bleeding (13.8% vs 12.2%; p = 0.882; Figure 1 ). The temporal distribution of bleeding events in the 2 study groups according to BARC classes is shown in Figure 2 .