Intermediate- or low-risk patients with severe aortic stenosis were excluded from earlier transcatheter aortic valve implantation (TAVI) clinical trials; however, they are already being treated by TAVI despite a lack of data regarding the safety and efficacy in these patients. We aimed to assess the safety and efficacy of TAVI in patients at intermediate or low risk. Patients undergoing TAVI during 2008 to 2014 were included into a shared database (n = 1,327). Procedural outcomes were adjudicated according to Valve Academic Research Consortium 2 definitions. Patients were stratified according to their Society of Thoracic Surgeons (STS) score into 3 groups: high (STS ≥8, n = 223, 17%), intermediate (STS 4 to 8; n = 496, 38%), or low risk (STS <4; n = 576, 45%). Low-risk patients were significantly younger and more likely to be men compared to intermediate- and high-risk patients. Baseline characteristics differed significantly between the groups with a gradual increase in the rates of previous bypass surgery, stroke, peripheral vascular disease, renal failure, lung disease, and frailty scores, from low to high risk groups. Compared with intermediate- and high-risk patients, low-risk patients were more likely to undergo TAVI through the transfemoral route (81% vs 88% vs 95%, p <0.001) and under conscious sedation (69% vs 72% vs 81%, <0.001). There were no significant differences in the rates of procedural complications apart from acute kidney injury (19% vs 17% vs 13%, p = 0.03) and stroke rates (4.5% vs 2% vs 2.3%, p = 0.1). Short- and long-term mortality rates were significantly higher for intermediate- (hazard ratio [HR] 1.9, 95% confidence interval [CI] 1.2 to 2.9) and high-risk patients (HR 4.1, 95% CI 2.7 to 6.4) than low-risk patients also after multivariate adjustment (HR 1.6, 95% CI 1 to 2.6 and HR 2.7, 95% CI 1.7 to 4.5, respectively; all p <0.05). In conclusion, TAVI for intermediate- and low-risk patients is safe and associated with improved outcome compared with high-risk patients.
The aim of the present study was to assess the procedural safety and outcome of a large cohort of lower risk, consecutive patients undergoing transcatheter aortic valve implantation (TAVI) compared to the patients undergoing high and inoperable TAVI in contemporary practice.
Methods
The study included consecutive patients with symptomatic, severe aortic stenosis (AS) who underwent TAVI from 2008 to 2014 at 1 of 3 tertiary centers in Israel: Sheba Medical Center, Rabin Medical Center (Beilinson), and Tel-Aviv Sourasky Medical Center ( Supplementary Table 1 ). The study was approved by the institutional review board of each of the participating centers. Eligibility for TAVI was established on the basis of the consensus of a multidisciplinary heart team, and even if the calculated Society of Thoracic Surgeons (STS) or EuroSCORE was low, they were deemed as high surgical risk on the basis of other factors and co-morbidities or frailty measurements not included in the standardized risk scores. For the purpose of this study, patients were divided according to their STS score into 3 groups: high risk (STS score >8), intermediate risk (STS score 4 to 8), and low risk (STS score <4).
Selection of the transcatheter heart valve, approach, and anesthesia was done at the discretion of the physician. Prespecified clinical and laboratory data were collected for all patients at baseline before the procedure, immediately after the procedure, during the index hospitalization, and during long-term follow-up. Collected data included medical history, electrocardiogram, echocardiography studies, laboratory tests, and clinical outcomes. Inhospital outcomes were collected according to the Valve Academic Research Consortium 2 consensus document and included length of stay, blood transfusion, acute kidney injury, periproce dural myocardial infarction, stroke, bleeding, vascular complications, and death. All suspected events were adjudicated by a blinded interventional cardiologist.
Statistical analyses were performed with SPSS version 21 (IBM Corporation, Armonk, NY). Categorical variables were reported as frequency and percentages and continuous variables as means and standard deviations (SD) or medians and interquartile ranges (IQR). Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test, Q-Q plots, and histogram. Categorical variables were compared using the chi-square test and continuous variables using analysis of variance (Scheffe method for post hoc analysis) or the Kruskal–Wallis test (Mann–Whitney for post hoc analysis). Univariate Cox regression was used to evaluate the association between low-, intermediate-, and high-risk categories and mortality. Multivariate Cox regression was used to evaluate the association while controlling for potential confounders. Age, gender, TAVI approach, kidney injury, bleeding, vascular complications, and risk categories were included in the first multivariate Cox regression block and variables with p value <0.2 in the univariate analysis were included in the second block with variable selection by backward likelihood ratio method. Kaplan–Meier plot was used to describe the mortality between categories and log-rank test to compare between them. A 2-tailed p values <0.05 were considered statistically significant.
Results
The study population included 1,327 patients. Of them, 43% were men, and median age was 83 years (79 to 86 years; Table 1 ). The median STS score of the study cohort was 4.4 (3.1 to 6.6) and the EuroSCORE II was 4.49 (2.61 to 7.4). Among the entire study population, the high risk (STS score >8%) group comprised the minority of the patients (n = 223; 17%), followed by the intermediate risk (STS score 4% to 8%) group (n = 496; 38%) and the low risk (STS score <4%) group (n = 576; 45%). Throughout the study period, the proportion of low-risk patients remained relatively stable, whereas the proportion of high-risk patients gradually decreased along an increase in the proportion of intermediate-risk patients ( Figure 1 ; p for trend <0.001).
| Variable | All (n=1,327) | Patient risk | P value | ||
|---|---|---|---|---|---|
| Low (n=576) | Intermediate (n=496) | High (n=223) | |||
| Age; median (IQR) (Years) | 83 (79-86) | 81 (77-85) | 84 (80-87) | 85 (80-88) | <0.001 |
| Males | 574 (43%) | 280 (49%) | 185 (37%) | 97 (44%) | 0.001 |
| Body mass index; median (IQR) (Kg/m 2 ) | 26 (24-29) | 27 (25-30) | 25.9 (23.6-29.3) | 24.9 (22.7-28.1) | <0.001 |
| Hypertension | 1162 (88%) | 489 (85%) | 449 (91%) | 202 (91%) | 0.008 |
| Diabetes mellitus | 470 (36%) | 161 (28%) | 207 (42%) | 95 (43%) | <0.001 |
| Hyperlipidemia | 1068 (81%) | 447 (78%) | 411 (83%) | 186 (83%) | 0.037 |
| Coronary artery disease | 666 (51%) | 279 (49%) | 254 (51%) | 121 (55%) | 0.34 |
| Prior Percutaneous coronary intervention | 437 (34%) | 209 (37%) | 162 (33%) | 66 (30%) | 0.16 |
| Prior coronary bypass | 264 (20%) | 87 (15%) | 103 (21%) | 70 (32%) | <0.001 |
| Prior stroke | 196 (15%) | 74 (13%) | 69 (14%) | 48 (22%) | 0.007 |
| Peripheral vascular disease | 147 (11%) | 36 (6.3%) | 52 (11%) | 54 (24%) | <0.001 |
| Prior atrial fibrillation | 398 (31%) | 148 (26%) | 152 (31%) | 94 (43%) | <0.001 |
| Prior pacemaker | 116 (8.9%) | 58 (10%) | 42 (8.6%) | 15 (6.8%) | 0.297 |
| Renal failure | 329 (25%) | 71 (12%) | 153 (31%) | 103 (47%) | <0.001 |
| Hemodialysis | 6 (1.5%) | 0 | 3 (1.8%) | 3 (5.4%) | 0.24 |
| Chronic lung disease | 249 (19%) | 79 (14%) | 97 (20%) | 71 (32%) | <0.001 |
| Oncological history | 103 (14%) | 52 (24%) | 34 (11%) | 16 (9%) | <0.001 |
| Past thoracotomy | 164 (23%) | 33 (15%) | 70 (23%) | 60 (34%) | <0.001 |
| Porcelain aorta | 36 (5.1%) | 13 (6.1%) | 13 (4.3%) | 10(5.7%) | 0.64 |
| Liver disease | 6 (0.9%) | 2 (0.9%) | 2 (0.7%) | 2 (1.2%) | 0.845 |
| Frailty | 156 (17%) | 48 (11%) | 56 (17%) | 51 (33%) | <0.001 |
| STS score (%); median (IQR) | 4.4 (3.1-6.6) | 2.9 (2.3-3.4) | 5.4 (4.7-6.4) | 10.3 (9.1-13.8) | <0.001 |
| EuroSCORE 1 (%); median (IQR) | 14.24 (9.2-23.6) | 10.1 (7.5-15.5) | 16.1 (11.4-24.7) | 26.9 (18.7-38) | <0.001 |
| EuroSCORE 2 (%); median (IQR) | 4.49 (2.61-7.4) | 3.1 (1.98-5.03) | 5.3 (3.6-8.7) | 8.1 (5.6-12.8) | <0.001 |
| Baseline echocardiographic data | |||||
| Impaired LV function | 303 (25%) | 104 (20%) | 117 (26%) | 74 (36%) | <0.001 |
| Mitral regurgitation >Mild | 206 (23%) | 63 (15%) | 80 (25%) | 62 (38%) | <0.001 |
| Aortic insufficiency >Mild | 131 (15%) | 36 (8.7%) | 64 (20%) | 31 (19%) | <0.001 |
| AV area (cm 2 ); median (IQR) | 0.7 (0.6-0.8) | 0.7 (0.6-0.86) | 0.7 (0.6-0.8) | 0.6 (0.5-0.8) | <0.001 |
| AV maximal PG (mmHg); mean±SD | 76±23 | 78±22 | 75±25 | 75±25 | 0.11 |
| AV mean PG (mmHg); mean±SD | 57±17 | 48±15 | 47±16 | 48±20 | 0.51 |
| Systolic pulmonary artery pressure (mmHg); mean±SD | 41±16 | 38±16 | 42±16 | 44±16 | <0.001 |
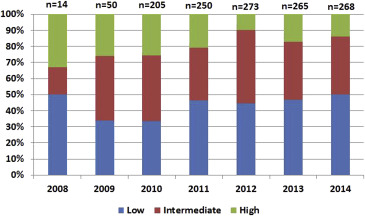
As detailed in Table 1 , baseline patient characteristics were significantly different among the 3 groups. Prevalence of renal failure, chronic lung disease, past thoracotomy, and frailty condition were also significantly higher in high-risk patients. Conversely, oncologic history and porcelain aorta were more frequent in low-risk patients. Baseline echocardiographic characteristics also differed between the 3 groups with greater frequency of impaired left ventricular function, concomitant mitral and aortic regurgitation, and elevated pulmonary artery pressures in high-risk patients ( Table 1 ).
The selection of transcatheter heart valve was not influenced by the patient’s risk; however, high-risk patients received smaller sized valves more frequently, whereas low-risk patients received larger sized valves. Additionally, valve-in-valve procedures were performed more frequently in high-risk patients. Access for TAVI was more frequently transfemoral in low-risk patients (95% vs 88% vs 81%, respectively, p <0.001) which was also associated with lower rates of general anesthesia (19% vs 28% vs 31%, respectively, p <0.001), compared to intermediate- and high-risk patients. Finally, patient’s risk did not influence the procedure fluoroscopy time, contrast use, or the procedural success which were comparable between all patient groups ( Table 2 ).
| Variable | All (n=1,327) | Patient risk | P value | ||
|---|---|---|---|---|---|
| Low (n=576) | Intermediate (n=496) | High (n=223) | |||
| Approach | |||||
| Transfemoral | 1160 (89%) | 544 (95%) | 428 (88%) | 181 (81%) | <0.001 ∗ |
| Transapical | 101 (7.7%) | 17 (3%) | 39 (8%) | 27 (12%) | |
| Other | 50 (3.8%) | 10 (1.8%) | 20 (4.1%) | 15 (6.7%) | |
| Anesthesia | |||||
| General | 301 (26%) | 93 (19%) | 121 (28%) | 62 (31%) | <0.001 ∗ |
| Conscious sedation | 853 (74%) | 402 (81%) | 310 (72%) | 138 (69%) | |
| Valve type | |||||
| SAPIEN XT | 398 (31%) | 169 (30%) | 151 (31%) | 62 (28%) | 0.25 |
| CoreValve | 893 (69%) | 396 (70%) | 325 (67%) | 160 (72%) | |
| Other | 13 (1%) | 5 (0.9%) | 8 (1.7%) | 0 | |
| Valve size (mm) | |||||
| 23 | 200 (16%) | 59 (11%) | 91 (19%) | 42 (19%) | <0.001 |
| 25 | 6 (0.5%) | 2 (0.4%) | 3 (0.6%) | 0 | |
| 26 | 637 (50%) | 268 (48%) | 247 (52%) | 109 (50%) | |
| 29 | 413 (32%) | 218 (39%) | 127 (27%) | 62 (28%) | |
| 31 | 25 (2%) | 12 (2.1%) | 8 (1.7%) | 5 (2.3%) | |
| Valve-in-valve procedure | 61 (4.6%) | 10 (1.7%) | 20 (4%) | 16 (7.2%) | 0.001 |
| Fluoroscopy time; median (IQR) | 15.5 (12.6-20) | 15.2 (12.9-19.8) | 16.1 (12.5-19.5) | 17.1 (12.6-22.8) | 0.64 |
| Contrast Volume; median (IQR) | 152 (121-188) | 150 (125-180) | 154 (120-191) | 150 (111-200) | 0.63 |
| Procedure success | 1251 (94%) | 544 (94%) | 467 (94%) | 209 (94%) | 0.924 |
Table 3 summarizes the procedural complications and inhospital outcomes according to patient STS risk score. Overall, the patients’ risk did not impact the immediate procedural outcome. There were comparable rates of procedural success ( Table 2 ; 94% vs 94% vs 94%, respectively, p = 0.9) and procedural mortality in patients with low, intermediate, and high risk (0.2% vs 0.8% vs 0.9% respectively, p = 0.27) despite a nonsignificant trend toward lower mortality in low-risk patients. Higher rates of valve malposition were documented in the low-risk group (6.4% vs 2.9% vs 1.7%, respectively, p = 0.03), but these higher rates did not translate into higher rates of a need for a second valve. Similarly, inhospital complications were overall comparable between the groups, including vascular complications, bleeding, tamponade, and periprocedural and postprocedural myocardial infarction. High-risk patients had twofold increase in stroke rates compared to lower risk patients without statistically significant difference (2.3% vs 2% vs 4.5%, respectively, p = 0.13); however, at 1-month follow-up, this difference reached statistical significance (2.6% vs 3.3% vs 7.9%, respectively, p = 0.003). The rates of acute kidney injury (13% vs 17% vs 19%, respectively, p = 0.03), the need for hemodialysis (0 vs 2.3% vs 5.7%, respectively, p <0.001), and inhospital fever and/or infections (5.7% vs 12% vs 17%, respectively, p <0.001) occurred more frequently in intermediate- and high-risk patients. This was also translated into slightly prolonged hospital stay. At 1-month echocardiographic follow-up, low-risk patients demonstrated higher left ventricular ejection fraction (58 ± 7% vs 56 ± 9% vs 56 ± 9%, respectively, p = 0.007) with no difference in moderate or worse perivalvular leak (9.7% vs 11.8% vs 14.6%, respectively, p = 0.3).
| Variable | All (n=1,327) | Patient risk | P value | ||
|---|---|---|---|---|---|
| Low (n=576) | Intermediate (n=496) | High (n=223) | |||
| Valve malposition | 26 (3.6%) | 14 (6.4%) | 9 (2.9%) | 3 (1.7%) | 0.03 |
| Need for 2 nd valve | 46 (3.6%) | 19 (3.4%) | 18 (3.8%) | 8 (3.7%) | 0.94 |
| Unplanned surgery | 7 (0.7%) | 3 (0.6%) | 2 (0.6%) | 1 (1%) | 0.9 |
| Coronary obstruction | 4 (0.4%) | 1 (0.2%) | 2 (0.6%) | 1 (1%) | 0.47 |
| Tamponade | 25 (1.9%) | 14 (2.4%) | 8 (1.6%) | 2 (0.9%) | 0.32 |
| Periprocedure MI | 11 (1.1%) | 2 (0.4%) | 6 (1.7%) | 2 (1.9%) | 0.114 |
| Spontaneous MI | 5 (0.4%) | 2 (0.3%) | 1 (0.2%) | 2 (0.9%) | 0.37 |
| Any stroke | 33 (2.5%) | 13 (2.3%) | 10 (2%) | 10 (4.5%) | 0.13 |
| Major/life-threatening bleeding | 98 (7.5%) | 46 (8%) | 34 (6.9%) | 17 (7.7%) | 0.447 |
| Any kidney injury | 203 (15%) | 73 (13%) | 84 (17%) | 43 (19%) | 0.033 |
| Hemodialysis | 14 (1.4%) | 0 | 9 (2.3%) | 6 (5.7%) | <0.001 |
| Any vascular complication | 280 (22%) | 124 (22%) | 108 (22%) | 47 (22%) | 0.97 |
| Pacemaker implantation | 241 (18.7%) | 100 (18%) | 95 (20%) | 41 (19%) | 0.723 |
| Any infection | 130 (10%) | 32 (5.7%) | 59 (12%) | 37 (17%) | <0.001 |
| Heart failure episode | 58 (6.8%) | 24 (7.7%) | 20 (5.9%) | 14 (7.6%) | 0.61 |
| Hospitalization days; median (IQR) | 5 (4-7) | 5 (4-6) | 5 (4-7) | 6 (4-9) | <0.001 |
| Mortality | |||||
| Procedural | 8 (0.6%) | 1 (0.2%) | 4 (0.8%) | 2 (0.9%) | 0.27 |
| In-hospital | 42 (3.2%) | 7 (1.2%) | 17 (3.4%) | 16 (7.2%) | <0.001 |
| 30-day | 45 (3.4%) | 9 (1.6%) | 16 (3.2%) | 17 (7.6%) | <0.001 |
| 1-year | 144 (10.9%) | 34 (5.9%) | 54 (10.9%) | 51 (22.9%) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


