33 Other Interventional Techniques
Occlusion of the Left Atrial Appendage (LAA Occlusion)
 Basics
Basics
Atrial fibrillation is the most common cardiac arrhythmia and the most common cause for rhythm-related hospital admissions. The prevalence is 1.5 to 2 % of the total population and is ~6 % of the population over 60 years of age, with increasing prevalence with advancing age.
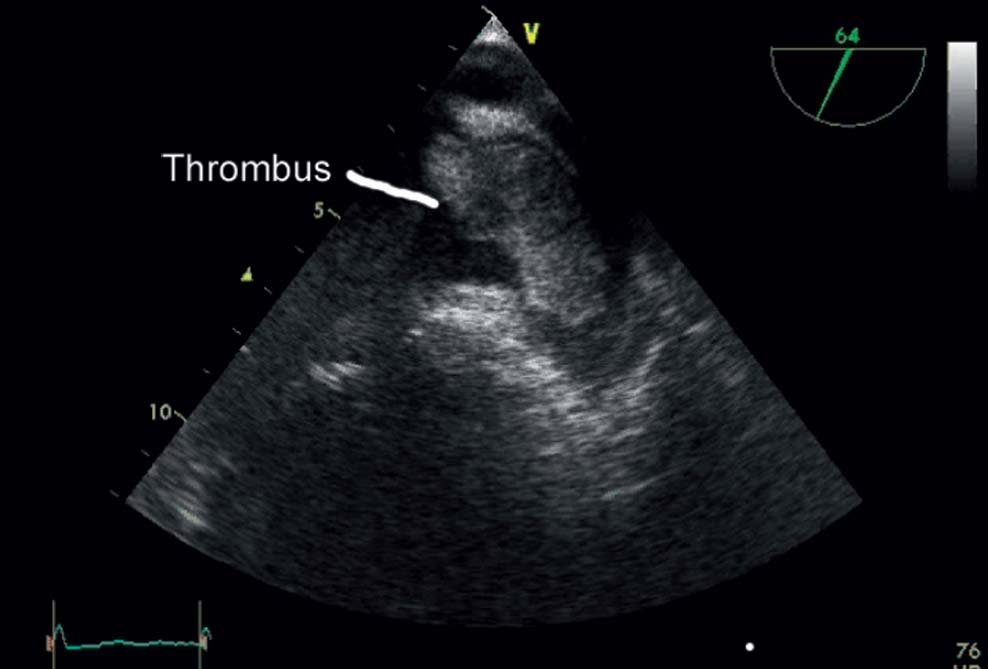
Patients with atrial fibrillation have a five-fold increased risk of stroke. The percentage of strokes due to atrial fibrillation is strongly age-dependent and increases from 1.5 % in 50- to 59-year-olds to 23.5 % in 80- to 89-year-olds. Stroke is the major cause of permanent disability with atrial fibrillation and is a frequent cause of death. More than 80 % of all strokes are thromboembolic events. Most thrombi form in the left atrial appendage (LAA). Thrombi from the left atrium are often relatively large (Fig. 33.1) and thus lead to strokes with substantial neurological deficits.
The most important treatment goals in atrial fibrillation are on the one hand antiarrhythmic therapy, either pharmacologically with antiarrhythmic agents or interventionally (e.g., pulmonary vein isolation), and on the other prevention of thromboembolism. The most important measure to prevent thromboembolism is pharmacological therapy with vitamin K antagonists or with newer anticoagulants, such as oral factor Xa antagonists and thrombin antagonists. Oral anticoagulation with vitamin K antagonists has been shown to reduce the stroke rate by two-thirds and mortality by about one-quarter. The newer oral anticoagulants gave similar results regarding stroke reduction, and rates of intracerebral hemorrhage were the same or lower. Large registries have shown that a high percentage of patients who have an indication for oral anticoagulation do not receive this treatment because of their numerous comorbidities and risk factors. Conversely, some patients who are not candidates for oral anticoagulation nevertheless receive this treatment. A special concern with vitamin K antagonists is the narrow therapeutic range (INR 2–3), and with thrombin antagonists and the factor Xa antagonists there is concern over the impact of renal dysfunction and interactions with other drugs. Patients especially at risk for bleeding complications are those who also require additional treatment with single or dual platelet aggregation inhibitors.
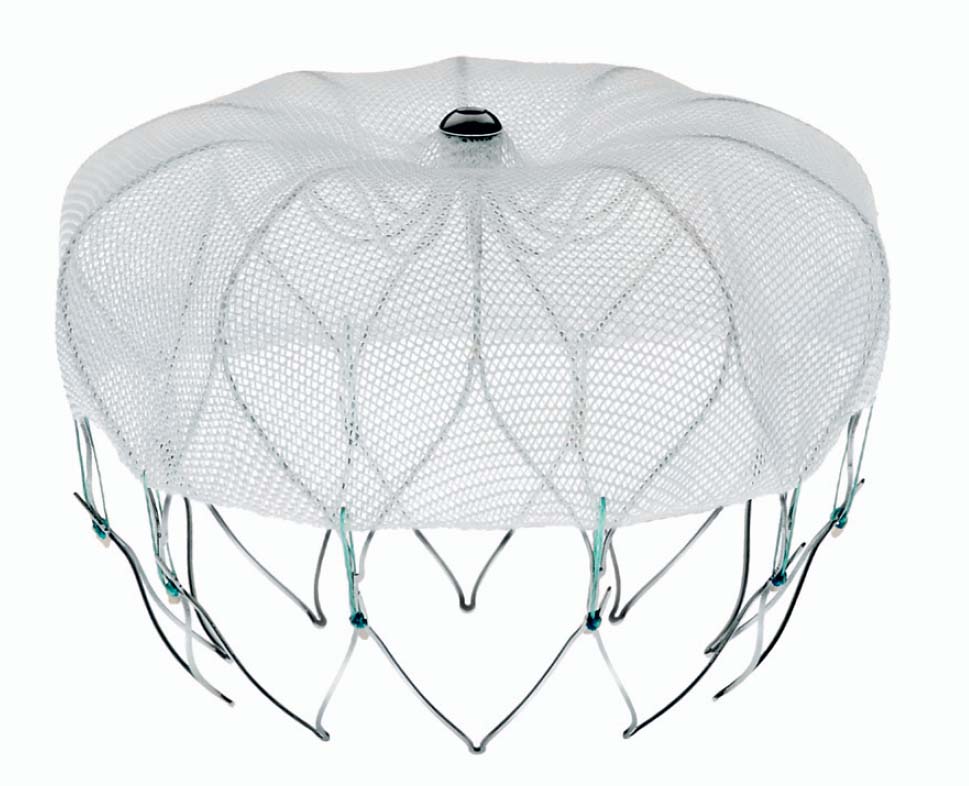
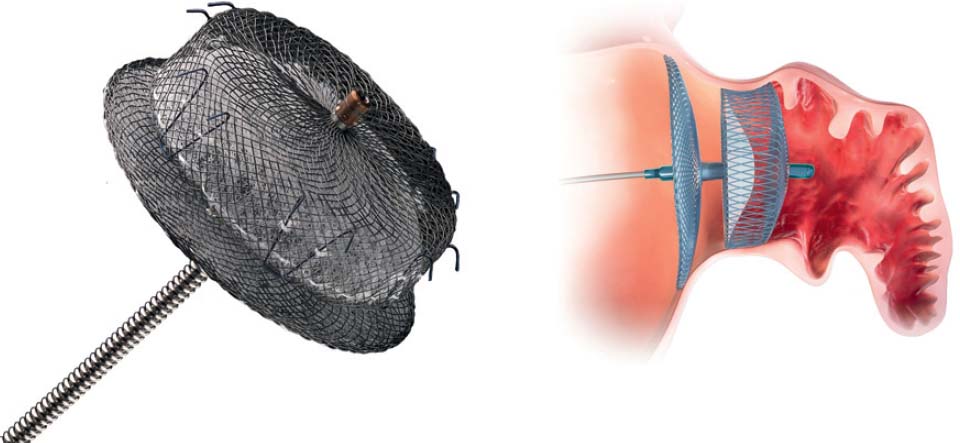
An alternative for patients at risk is interventional occlusion of the LAA. Currently, two systems are available for clinical use: the Watchman device (Boston Scientific, Natick, MA, USA) (Fig. 33.2) and the Amplatzer Cardiac Plug (St. Jude Medical, St. Paul, MN, USA) (Fig. 33.3). Results from randomized studies are available for the Watchman device; a randomized trial is ongoing for the Amplatzer Cardiac Plug; registry data are available for both devices.
Some major characteristics of the two systems are shown in Table 33.1.
 Indications and Contraindications
Indications and Contraindications
Interventional LAA occlusion is indicated in patients with atrial fibrillation who are at high risk for thromboembolic complications but have contraindications for chronic oral anticoagulation with vitamin K antagonists and new anticoagulants.
Thus, an interventional LAA occlusion should be considered for patients with a CHA2DS2-VASc score ≥ 2 and recurring bleeding complications (especially intracerebral hemorrhages), severe chronic kidney disease, need for dual platelet inhibition (e.g., after acute coronary syndrome, PCI with stent, etc.), and substantially limited compliance with taking medication.
 Preliminary Tests
Preliminary Tests
The most important preliminary test prior to LAA occlusion is transesophageal echocardiography (TEE) by an experienced examiner who also knows the LAA occlusion procedure.
The issues that need to be addressed during the examination are
 Exclusion of thrombi in the LAA
Exclusion of thrombi in the LAA
 Anatomy and morphology of the LAA
Anatomy and morphology of the LAA
– Measurement of the diameter of the LAA ostium and of the neck in at least four planes (0°, 40–60°, 90° and 130–180°)
– Number of lobules of the LAA
– Depth of the LAA
– Degree of trabecularization
– If possible three-dimensional visualization: round versus oval ostium
 Anatomy of the interatrial septum
Anatomy of the interatrial septum
 Procedure
Procedure
The procedure is preferably done under general anesthesia or deep sedation with continuous TEE monitoring during the placement phase. The anesthesiologist, echo-cardiographer, and interventionalist should work together as a good team. The anesthesiologist and echocardiographer should be protected from radiation during fluoroscopy by mobile lead protection. The procedure should be done in a catheterization laboratory with sufficient space. Ideally, all active participants would be able to follow the procedure on their own monitors. It is obligatory to provide the online echocardiogram on the display screen system for the interventionalist.
Table 33.1 Characteristics of the Watchman and Amplatzer devices for LAA occlusion
| Watchman | Amplatzer Cardiac Plug |
Material | Nitinol frame | Nitinol mesh, proximal disk, and distal lobe with compliant connection |
Sizes | 21, 24, 27, 30 and 33 mm | 16-, 18-, 20-, 22-, 24-, 26-, 28- and 30 mm lobe diameter |
Fixation | 10 fixation anchors arranged around the device perimeter | 10 circularly arranged hooks |
Membrane | Dacron membrane with 160-µm pore size | One polyester patch each in the disk and in the lobe |
Sheath size | 14F outer and 12F inner diameter With two-fold curve (predominant use) or one-fold curve | 9F, 10F and 13F sheath size, depending on device diameter With two-fold curve |
Preparation | Preassembled 12F delivery system | Assembly during the procedure, after the size has been determined |
Criteria for release | Position (echo and contrast media injection) Stability (gentle retraction and release) Seal (no residual flow around the device) Minimally 8%, maximally 20% compression | Position (echo and contrast media injection) Seal (no residual flow around the device) Lobe should be slightly compressed, lobe at a right angle to the LAA axis, separation of the lobe from the disk and concave form of the disk |
– General preparation for anesthesia
– Placement of the bladder catheter
– Antibiotic as a periprocedural single administration
– Generous sterile draping with access to both groins
– Placement of the TEE probe
– Capability to measure ACT
 Specific procedures:
Specific procedures:
– Arterial puncture with 4F or 5F and placement of a pigtail catheter in the aortic root for orientation during the transseptal puncture and for hemodynamic monitoring
– Venous puncture preferably of the right common femoral vein with a standard sheath (e.g., 6F)
– Administration of heparin (2,500–5,000 IU according to institutional policy) before transseptal puncture
– Placement of a 0.0032-in. wire in the superior vena cava
– Advancement of the sheath for transseptal puncture to the superior vena cava
– Transseptal puncture with puncture needle and sheath, if necessary under TEE guidance, preferably posteriorly. Advancement through a patent foramen ovale (PFO) should be avoided as this may complicate coaxial alignment of the sheath with the LAA axis
– Removal of the puncture needle and dilator and advancement of a 0.0035-in. standard wire
– Administration of unfractionated heparin at a therapeutic dose (ACT 250 seconds; regular ACT measurements during the procedure and adjustment of the anticoagulation)
– Advancement of a 5F or 7F pigtail catheter via the sheath and atraumatic positioning in the LAA
– Visualization and measurement of LAA dimensions
– By TEE in multiple planes (see above)
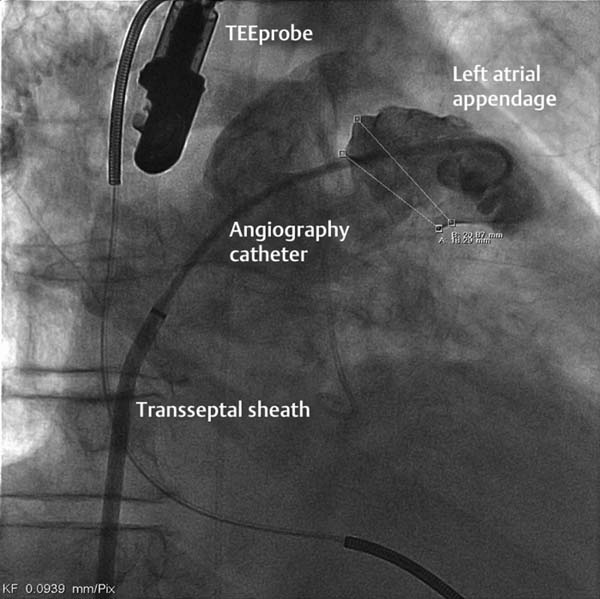
– Angiographically, usually in 30° RAO with cranial or caudal angulations (variable; see Fig. 33.4)
– Selection of the occluder size, ~2 to 4 mm larger than the measured diameter of the landing zone of the occluder
– Also consider the depth of the LAA when selecting the occluder, as both systems require a sufficient depth, which varies with the morphology
– Preparation of the occluder with sufficient purging of air from the system
– Placement of the implantation sheath into the LAA
– For the Watchman occluder via the pigtail catheter
– For the Amplatzer Cardiac Plug Occluder via a stiff wire with a short but very soft tip
– Advancement of the occluder within the sheath preferably under fluoroscopy to
– Control the movements of the sheath
– Detect air
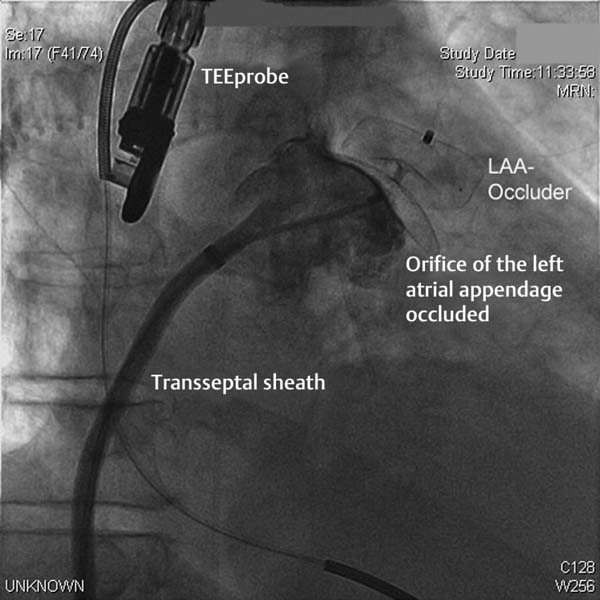
– Deployment of the occluder in the LAA under angiographic and echocardiographic monitoring
– Thorough verification of the correct placement of the occluder by
– TEE with multiple angulations, especially the location in the landing zone and in the ostium of the LAA
– TEE with measurement of the compression
– TEE and duplex color ultrasound visualization
– Angiography with contrast to visualize possible residual flow into the LAA
– Fluoroscopy to assess the morphology of the occluder
– Fluoroscopy for a possible pull test (for the Watchman always)
– After thorough verification and documentation of a good result, and when the release criteria are met (see Table 33.1), then release by turning the “delivery cable” (Fig. 33.5)
– If the positioning is inadequate, then do a partial pullback into the sheath and reposition
– If the result is good, then remove the sheath, under TEE monitoring as appropriate
– Apply a light pressure bandage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 General procedures:
General procedures: